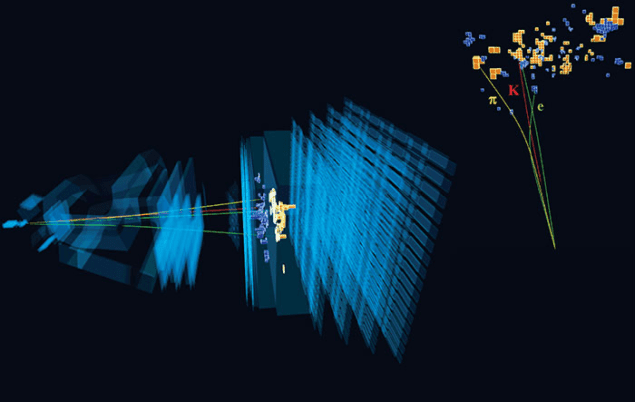
Electrons in twisted graphene ‘freeze’ when heated
21 Apr 2021 Isabelle Dumé
When most solids are heated, they melt into liquids. This behaviour makes sense in terms of entropy, or disorder: a liquid state is usually more disordered than a solid state, and higher temperatures mean that the particles within a material vibrate randomly with more energy. One exception, however, is helium-3. This isotope of helium solidifies on heating, because in its solid state, fluctuations in its atoms’ nuclear spin (or internal “rotation”) give it a higher entropy than its liquid counterpart. This phenomenon is known as the Pomeranchuk effect after the theoretical physicist Isaak Pomeranchuk, who predicted it in 1950.