Elekta Unity receives 510(k) clearance
06 Dec 2018 Tami Freeman
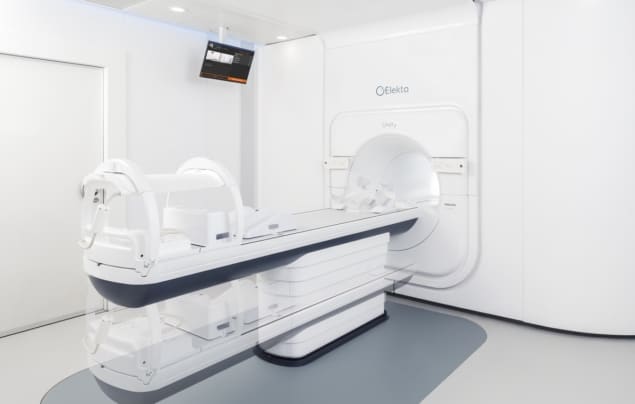
The Elekta Unity MRI-guided radiotherapy system has received 510(k) premarket notification from the US Food and Drug Administration, clearing the technology for commercial sales and clinical use in the US. Elekta Unity combines high-field 1.5 T MR imaging, precision radiation therapy and intelligent software, allowing clinicians to see what they treat in real time.
“Since receiving CE mark in June 2018, Elekta Unity has been transforming the care of cancer patients in Europe, and we are excited that this cutting-edge technology is now commercially available to US patients,” says Richard Hausmann, president and CEO of Elekta. “With Elekta Unity, it is now feasible to develop personalized, precision radiation therapy regimens that are optimized for safety and efficacy and make radiation therapy a viable treatment option for more patients.”
Unity has the potential to transform how clinicians treat cancer by enabling the delivery of the radiation dose while simultaneously visualizing the tumour and surrounding healthy tissue with high-quality MR images. Unity also integrates advanced tools that allow clinicians to adapt the patient’s treatment to this current anatomical information within a treatment session.READ MORE

“Unity is a tremendous leap forward in our ability to tailor radiation therapy to each patient’s tumour and anatomy and to adapt treatment in real time as the tumour changes shape and position relative to organs at risk,” says Christopher Schultz, chair of the Elekta MR-linac Consortium. “I believe this enabling technology will fundamentally transform how radiation therapy regimens are developed, implemented and adapted to achieve optimal outcomes for our patients. We are excited to offer Unity to our patients and are proud of our contributions to making this technology a clinical reality.”

15/12/2018 from physicsworld.com
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου