Moulded fabrication delivers practical platform for drug screening
30 Dec 2018 Belle Dumé
Researchers in Japan have demonstrated a novel collagen-based device that offers a promising alternative to animal testing for assessing the efficacy and toxicity of new drug formulations. The 3D structure, which comprises a double-layer collagen tube attached at both ends to silicone tubes, is easy to fabricate using only moulding techniques and can readily be perfused with cell cultures via an external pump (Biofabrication 11 015010).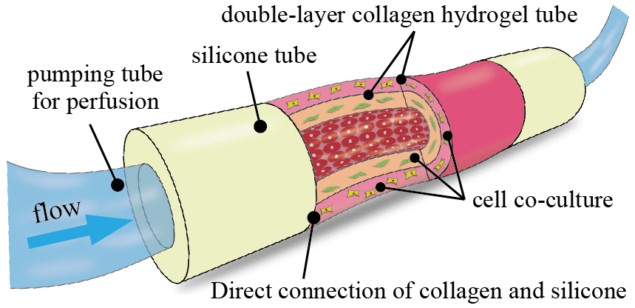

The moulded microtube structure overcomes many of the problems normally associated with in vitro 3D tissue models – which typically consist of cells embedded in a scaffold material. Such models also include vascular-like networks, which play an important role in biochemical reactions and the absorption of drugs into the human body.
Various techniques have been used to make these 3D structures, including bioprinting and soft lithography, but they tend to be complicated. It can also be difficult to connect these tissue models to external perfusion systems because it is no easy task to join hard pumping tubes to soft biological tissue. What’s more, the structures that have been created so far have mostly only had one scaffold layer, while multiple layers are need to reliably mimic complex biological structures.
With the new device, developed by Hiroaki Onoe at Keio University in Japan, successive collagen layers can be built up by repeated moulding. “We can also flexibly design the thicknesses of the collagen layers in the device and co-culture heterogenous cell types in the concentration we want in each collagen layer or surface,” explains Shun Itai, a graduate student in Onoe’s group. “And while the collagen tube is directly attached to silicone tubes, the collagen tube can easily be connected to an external pump for perfusion.”
Strong and stable
The device is strong enough to picked up with tweezers, says Itai, and is stable for more than three months in a culture medium. Cell cultures can be perfused into the device using a syringe pump that can be operated at different speeds.
“A collagen tube device such as ours needs to be designed with the target tissue model in mind,” adds Itai. “In this work, we targeted blood vessels as simple tissue models for testing the compatibility and perfusion capability of our device.”
The techniques used in this work could help advance the fabrication of simple-to-make in vitro vascular tissue models, Itai and Onoe tell Physics World. “These models could serve as a pharmacokinetic platform for drug testing, regenerative medicine and general biomedical research that does not involve experiments on animals.”The researchers chose to model medium-sized blood vessels with diameters of about 100–1000 µm – which is similar in size to arteries and veins. “We can change the size of our device by changing the dimensions of the mould used to make it,” Itai continues. “We have prepared several sizes of moulds for fabricating single- or double-layer collagen devices with inner diameters of 300–1000 µm.”
The researchers, who report their work in the journal Biofabrication, say they will now be developing a method to connect the individual collagen tube devices they have made to different tissue models. This would offer a way to construct multi-organ-on-a-chip models.
Read our special collection “Frontiers in biofabrication” to learn more about the latest advances in tissue engineering. This article is one of a series of reports highlighting high-impact research published in Biofabrication.

Belle Dumé is a contributing editor to Physics World
4/1/2019 from physicsworld.com
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου