‘Optical plucking’ manoeuvres single gold atoms into chemical reactions
25 Jul 2022
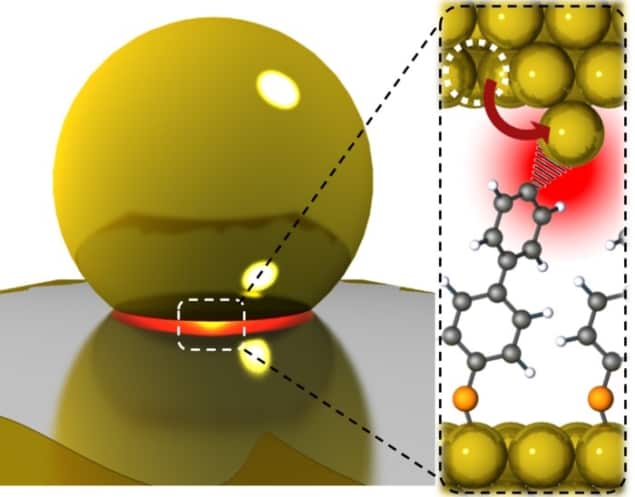
Plucky atoms: illustration of how light can be used to remove individual gold atoms from a surface. (Courtesy: Baumberg and collegues/Cavendish Laboratory/Department of Physics/University of Cambridge)
A new phenomenon whereby the energy from light confined to a nanocavity can “pluck” atoms from a gold surface, allowing them to form complexes with functional groups, has been discovered by researchers in the UK. Their experimental work is not readily transferable to industrial chemistry, but it could provide invaluable insights for research and development in multiple areas of science.
Transient bonds between molecules and metal surfaces are vital to areas such as electrochemistry, molecular electronics and most importantly catalysis. Researchers know that light can assist this process, but the exact mechanistic details are uncertain.
“You’ve got a surface science community, and these are people who do scanning tunnelling microscopy, often at low temperatures, looking at an adsorbed atom on a surface,” explains Jeremy Baumberg of the University of Cambridge. The positions of molecules can be tracked but, Baumberg points out, “you don’t see any chemical functionality typically”. He adds, “From the other end you have chemists, and they’re interested in the reaction of the molecules against a surface, but they don’t really know how that’s happening.”
Functionalized gold surfaces
In their new experiments, Baumberg and colleagues covered gold surfaces functionalized by various molecules with bare gold nanoparticles, creating 1 nm-thick nanocavities in the gap at the interfaces between the nanoparticle facets and the planar surfaces. They then irradiated the nanoparticle-covered surfaces with 633 nm light. Light interacts strongly with the gold surfaces through the plasmonic interaction, in which the oscillating electromagnetic field of the light couples to the oscillating electron density in a metal. This creates a strong electromagnetic field in the nanocavity.
Surface-enhanced Raman spectroscopy showed that, within this nanocavity, atoms could occasionally jump out of the gold surface, leaving behind an even smaller “picocavity” and a flare in the electromagnetic field intensity. “Five years ago, we would have told you that you were insane if you believed that you could confine light to the scale of a single atom,” says Baumberg.
The researchers considered whether the electromagnetic field gradient of the optical forces themselves could be responsible for this phenomenon, which they have termed optical plucking. They concluded, however, that these forces would be far too weak. Similarly, a simple optical heating model would require that the light heat the gold surface to around 12,000 K, whereas observations suggest it remains at room temperature.
Lightning rods
In the model that best fits their observations, the molecule behaves as a lightning rod, and the light decreases the energy barrier for the atom to escape by channeling electrons into the gold surface: “It doesn’t decrease it to completely zero, but it decreases it enough that there’s a chance that, just from the random thermal vibrations around, the atom can leap over that barrier and into the molecule’s clutches.”
In a paper published in Science Advances, the researchers describe how they studied non-reactive functional groups and found that the picocavities could sometimes persist for over a minute, although the flares died down much sooner. “We’ve been investigating a whole zoo of other molecules, and particularly if the molecules can undergo chemical reactions you see much more complicated things going on,” explains Baumberg. “We were trying here to have the [most stable] system we could. Even when there’s no ostensible chemical reaction, this molecule is still doing something to this metal surface.”READ MORE

Theoretical physicist Peter Nordlander of Rice University in Texas is impressed with the team’s accomplishments. “Baumberg has seen these picocavities and flares in previous experiments,” he says, “but he couldn’t say anything about important properties like the formation time or the lifetime because individual molecules can sit parallel to a surface or tilt, so he had five or six spectra and they were all a little bit different…What is new here is the development of a very clever experimental method to take a massive amount of spectra very quickly so he can do averaging and the very clever application of statistical analysis to extract these very central concepts.”
Nordlander suggests, however, that the requirement to put the molecule in a nanocavity means that the set-up used here is unlikely to be directly useful in industrial chemistry, and Baumberg agrees. “The really nice thing is that we now have tools to start seeing what’s going on in these tightly confined spaces,” says Baumberg. “But we can’t do theory for this yet – there’s theory in the paper, but it’s a bit of cobbled together classical and quantum theory: colleagues tell me we’re likely 10 years off being able to do these calculations properly.”
Tim Wogan is a science writer based in the US.
from physicsworld.com 29/7/2022
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου