A spoonful of sugar makes the dendrites go down
05 Sep 2022 Isabelle Dumé
A sucrose-modified aqueous electrolyte increases the mobility of zinc ions in response to the electric field and successfully achieves dendrite-free zinc batteries. (Courtesy: Nano Research, Tsinghua University)
Aqueous zinc batteries are promising alternatives to their lithium-ion cousins, but they suffer from one of the same problems: the formation of dendrites. These needle-like structures form on the surface of the zinc anode and grow into the electrolyte, causing the battery to short or, in some cases, even ignite. A team of researchers in China has now shown that adding ordinary table sugar (sucrose) chemically modified with hydroxyl groups to the electrolyte can slow down the growth of zinc dendrites by changing the solvent environment. What is more, the sucrose also forms a protective coating on the anode and slows down its corrosion.
Lithium-ion batteries are the most widely employed batteries today in portable electronics and electric vehicles, but the flammable and toxic organic electrolytes they contain are a cause for concern. Lithium is also expensive compared to some other, more common metals, and the global supply is victim to various uncertainties. Zinc batteries, which are normally formed with aqueous electrolytes, are an attractive substitute because zinc is cheaper, less toxic, more easily recycled and more widely available than lithium. They also have a high energy density, with a high specific capacity (820 mAh/g and 5 855 mAh/cm3) and a favourable redox potential (−0.76V versus the standard hydrogen electrode) of the Zn anode.
The problem is that when the zinc ion (Zn2+) concentration on the surface of the anode drops to zero, dendrites begin to grow on it. The presence of these structures causes the battery’s electrochemical performance to deteriorate and can be dangerous if left uncontrolled.
Modifying the solvent environment
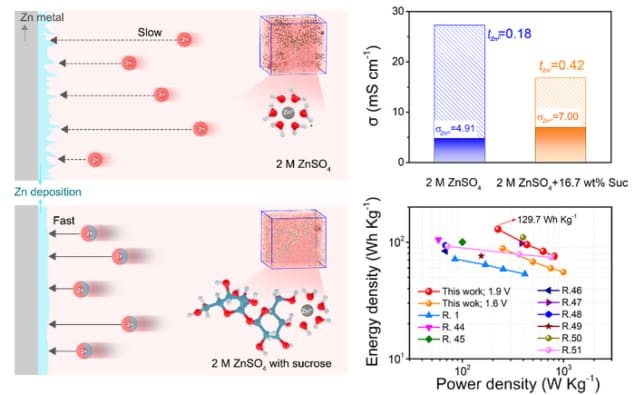
Recent studies have shown that modifying the solvent environment (or “solvation structure”) by, for example, introducing salts or including fewer water molecules, can increase the speed at which Zn2+ ions move in response to an electric field and therefore suppress dendrite growth. However, such adjustments unfortunately decrease the ionic conductivity of the battery system, leading to poorer overall performance.
In the new study, researchers led by nanotechnology expert Meinan Liu of the University of Science and Technology of China found that introducing sucrose containing hydroxyl groups is an effective way of regulating the solvation structure of Zn2+ ions, which enhances the speed at which the ions propagate without decreasing ionic conductivity. The sucrose can also stabilize the aqueous electrolyte while at the same time absorbing onto the Zn anode to form a protective layer on it. This impedes the corrosion of the electrolyte on the Zn anode, they say.
“Sucrose with hydroxyl groups strongly interacts with Zn2+ compared to water molecules in the electrolyte,” explains Liu. “It can therefore replace some of the water molecules and coordinate with Zn2+, so regulating the solvation structure of the ions.”
Dendrite formation reduced
“The modified Zn2+ solvation structure has an important influence on the kinetics of the ions, including the rate at which they diffuse through the electrolyte,” she tells Physics World. “Our experimental results clearly demonstrate that the transference number of Zn2+ ions increases with the introduction of sucrose. This enhanced mobility of the ions helps reduce the formation of dendrites as mentioned.”READ MORE

According to the researchers, their technique could help scientists develop high-performance Zn batteries and brings a safe, environmentally-friendly, Zn battery closer to reality.
Looking ahead, Liu and colleagues say they plan to focus on developing electrolytes with good ionic conductivity that work at lower temperatures. They detail their present study in Nano Research.

Isabelle Dumé is a contributing editor to Physics World
from physicsworld.com 7/9/2022
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου