Researchers ‘tattoo’ gold nanopatterns onto live cells
05 Sep 2023 Tami Freeman
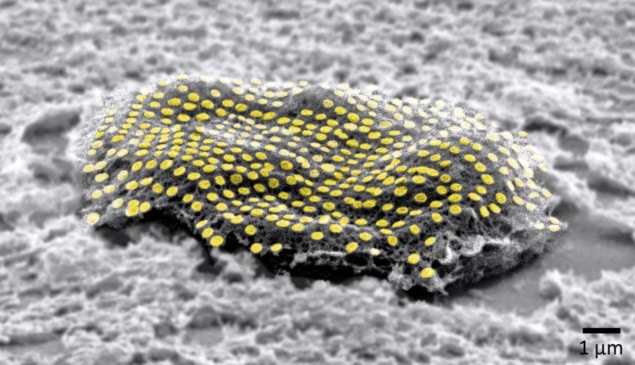
Printing nanopatterns False-coloured gold nanodot array on a living fibroblast cell. (Courtesy: Kam Sang Kwok and Soo Jin Choi, Gracias Lab/Johns Hopkins University)
The ability to merge electronics and optical sensors with the human body at the single-cell level could one day enable remote monitoring and control of individual cells in real time. Advances in electronics fabrication have made it possible to create transistors and sensors with nanoscale resolution, while innovative nanopatterning techniques enable assembly of these devices on flexible substrates. Such processes, however, generally require harsh chemicals, high temperatures or vacuum techniques that are unsuitable for living cells and tissues.
To overcome these obstacles, a research team at Johns Hopkins University has developed a non-toxic, high-resolution and cost-effective process for printing gold nanopatterns onto living tissue and cells. Reporting their findings in Nano Letters, they demonstrate that the new technique can “tattoo” living cells and tissues with flexible arrays of gold nanodots and nanowires. Ultimately, the method could be used to integrate smart devices with living tissue for applications such as bionics and biosensing.
“If we had technologies to track the health of isolated cells, we could maybe diagnose and treat diseases much earlier and not wait until the entire organ is damaged,” explains team leader David Gracias in a press statement. “We’re talking about putting something like an electronic tattoo on a living object tens of times smaller than the head of a pin. It’s the first step towards attaching sensors and electronics on live cells.”
Gracias, Luo Gu and colleagues have designed a three-stage nanotransfer printing process to bond gold nanopatterns to live cells. In the first step, they used conventional nanoimprint lithography (NIL) to print arrays of gold nanodots or nanowires onto polymer-coated silicon wafers. They then dissolved the polymer, freeing the nanoarrays for transfer onto glass coverslips.
Next, the researchers functionalized the gold surface with cysteamine and coated the gold NIL-arrays with an alginate hydrogel transfer layer. They showed that this approach could reliably transfer 8 × 8 mm arrays of nanodots and nanowires from the glass onto the soft and flexible hydrogels. In the final step, the gold NIL-arrays are conjugated with gelatin to enable their transfer onto living cells or tissue. Dissociating the hydrogel transfer layer then exposes the gold pattern.
The researchers investigated the behaviour of live fibroblast cells seeded onto arrays of 250 nm-diameter gold dots (550 nm centre-to-centre spacing) or 300 nm-wide gold wires (450 nm spacing) on alginate hydrogels. Around 24 h after seeding, cells on the nanowire-printed hydrogel preferably migrated parallel to the nanowires, whereas those on the nanodots exhibited random, but slightly faster, migration. Cells on the nanowires also exhibited roughly twice the elongation of those on the nanodots. These findings demonstrate the ability of the gold NIL-arrays to guide cell orientation and migration. Biocompatible process Gold nanowire array printed on a rat brain. (Courtesy: Kam Sang Kwok and Soo Jin Choi, Gracias Lab/Johns Hopkins University)
Biocompatible process Gold nanowire array printed on a rat brain. (Courtesy: Kam Sang Kwok and Soo Jin Choi, Gracias Lab/Johns Hopkins University)
 Biocompatible process Gold nanowire array printed on a rat brain. (Courtesy: Kam Sang Kwok and Soo Jin Choi, Gracias Lab/Johns Hopkins University)
Biocompatible process Gold nanowire array printed on a rat brain. (Courtesy: Kam Sang Kwok and Soo Jin Choi, Gracias Lab/Johns Hopkins University)As well as being biocompatible with cells and tissues, alginate hydrogel can also transfer gold NIL-arrays onto living organs and cells. To demonstrate this, the researchers positioned nanowire-printed hydrogels on the cerebral cortex of a whole brain and a coronal brain slice.
After 2 h in culture media and dissociation of the hydrogel, the nanowires remained bonded to the surface of the whole brain. In contrast, nanowires on the brain slice did not adhere, suggesting that adhesion strength varies among different cell types and culture methods. The researchers note that further studies are needed to characterize and optimize adhesion mechanisms for robust long-term bonding.
Finally, to assess biotransfer printing at the single-cell level, the researchers cultured monolayer cell sheets on gold NIL-array-printed alginate hydrogels. After 24 h, they flipped over the fibroblast-seeded hydrogels onto gelatin-coated coverslips and let the cells attach to the coverslips overnight.
After dissociating the alginate hydrogel, fluorescence microscopy revealed that fibroblasts patterned with gold nanodots had a viability of approximately 97%, while those patterned with nanowires had a viability of approximately 98%, indicating that the printing process is biocompatible with live cells. Reflective colours seen on the patterned fibroblast cell sheet suggest that the shape of the gold NIL-array was retained.
The fabrication process is also compatible with microscale photolithography, which enabled the researchers to create 200 µm wide hexagonal and triangular patches of gold NIL-arrays. They then biotransfer printed these onto cell sheets, leading to selective growth of fibroblast cells on the micropatches. Movies recorded over 16 h showed that cells with patches of nanowires printed on top appeared healthy and able to migrate, with the arrays remaining on the soft cells even while they moved.READ MORE

“We’ve shown we can attach complex nanopatterns to living cells, while ensuring that the cell doesn’t die,” says Gracias. “It’s a very important result that the cells can live and move with the tattoos because there’s often a significant incompatibility between living cells and the methods engineers use to fabricate electronics.”
Gracias and colleagues conclude that their nanopatterning process, combined with standard microfabrication techniques, “opens up opportunities for the development of new cell culture substrates, biohybrid materials, bionic devices and biosensors”. Next, they plan to try to attach more complex nanocircuits that can stay in place for longer periods, as well as experimenting with different types of cells.

Tami Freeman is an online editor for Physics World
FROM PHYSICSWORLD.COM 6/9/2023
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου