Superconducting nanowire detectors accurately estimate blood flow in the brain
20 Sep 2021 Jigar Dubal
The amount of blood flowing through the cerebral arteries and veins can strongly influence brain function, as it impacts both oxygen delivery and removal of waste products from the brain. As such, medical professionals utilize cerebral blood flow (CBF) as a marker of a patient’s cerebrovascular function.
One common technique, diffuse correlation spectroscopy (DCS), utilizes near-infrared light to monitor CBF. In DCS, a near-infrared laser with a long coherence length illuminates the tissue. As the light interacts with moving red blood cells within the arteries and veins, it undergoes scattering. The DCS system detects this scattered light and analyses it to determine the CBF.
Current DCS systems typically use single-photon avalanche photodiodes (SPADs) with a 700–850 nm light source and a source–detector separation of 25 mm. These detectors have a low signal-to-noise ratio (SNR), however, which hinders their ability to accurately determine CBF measurements. This, in turn, precludes the high acquisition rates required to determine dynamic blood flow changes. To improve brain sensitivity and reduce scalp signal contamination, ideal conditions include large source–detector separations, longer wavelengths of light (1000 nm or above) and fast acquisition rates, all of which are difficult to achieve with current SPAD systems.
As an alternative, the authors of a new paper, published in Neurophotonics, propose using superconducting nanowire single-photon detectors (SNSPDs) to improve current DCS methods. SNSPDs consist of a thin film of superconducting material with high single-photon sensitivity and detection efficiency. The authors believe that this method could provide more robust measurements of CBF.
SNSPD advantages
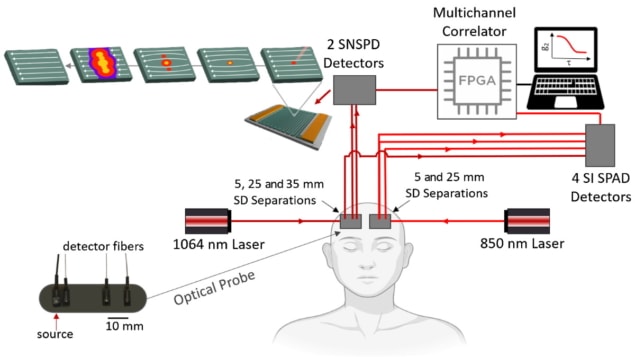
The paper’s first author, Nisan Ozana, alongside a team at Harvard Medical School, Massachusetts General Hospital and MIT Lincoln Labs, has developed DCS systems that use Quantum Opus SNSPDs and a 1064 nm light source. To assess this new approach, they obtained CBF measurements from 11 participants using both SPAD- and SNSPD-based systems, comparing the results obtained from the two systems.
The researchers demonstrated a sixteen-fold improvement in SNR when obtaining CBF measurements using SNSPD-based systems, in comparison with typical SPAD-based DCS systems (operating at 850 nm). This was partly due to a seven to eight times increase in the number of photons available at the detector (operating at 1064 nm) using SNSPD. Additionally, SNSPD demonstrated an increased detection efficiency (88%) compared with SPAD (58%). The significant increase in SNR allowed faster acquisition rates (20 Hz) than SPAD-based systems (1 Hz) at the same source–detector separation, resulting in clear detection of arterial pulses.
The team also found that increasing the source–detector separation to 35 mm improved brain blood flow sensitivity by 31.6% in the CBF measurements. Further, the researchers examined physiological changes during breath-holding and hyperventilation. Measurements obtained during breath-holding displayed the expected relative increase of 69% in CBF. During hyperventilation, they measured a 16.5% relative reduction in CBF, as expected due to the known increase of blood flow into the scalp and decrease of blood flow to the brain. Both results agreed well with previous MRI and PET studies.
The future of SNSPD
The ability to improve the accuracy of CBF measurements using SNSPD-based DCS systems is clear. “While current commercial SNSPDs are expensive, bulky and loud, they may allow for more robust measures of non-invasive cerebral perfusion in an intensive care setting,” say the researchers.READ MORE

For future implementation, the increased brain sensitivity observed for measurements at larger source–detector distances could be utilized to recover CBF changes more consistently across subjects. “This is needed to provide better accuracy and consistent efficacy when moving to adult clinical applications,” Ozana explains.

Jigar Dubal is a PhD contributor to Physics World. Jigar works in the Centre for Vision, Speech and Signal Processing at the University of Surrey, where he uses optical imaging techniques to characterize tumour response during radiotherapy. Find out more about our student contributor networks
from physicsworld.com 25/9/2021
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου