Small-world networks regulate transcription in cells
19 Oct 2021 Simon Lichtinger
The regulatory patterns that underpin gene expression may originate from the spatial organization of the genome, according to a new study reported in Nature Communications. A collaborative research team, from the universities of Edinburgh, Göttingen and Oxford, has used simulations of a simple DNA model to provide insight into the mechanisms of this genetic control.
At the interface of cellular information storage and protein synthesis lies transcription, the process of copying DNA for subsequent translation into proteins. With more than 20,000 genes present in human cells, however, there is a need for tight regulation of this process. One such regulation mechanism involves transcription factors, which bind to promoters and initiate transcription by coupling to distant enhancers, forming a structural loop.
Experimentally, researchers employ genome-wide association studies to establish correlations between thousands of genes. Computer simulations can help link this whole-genome perspective to observations from classic experiments, for example by rationalizing how the addition of just a few proteins can drastically alter cell physiology.
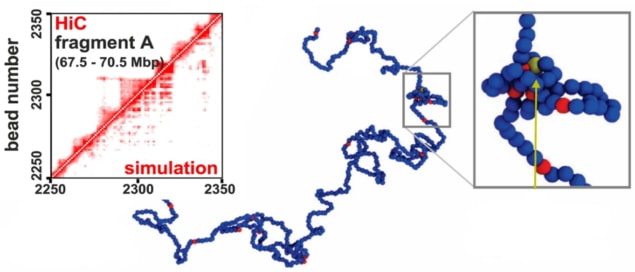
“This is where the experience with this [DNA] model in the past helped a lot,” says corresponding author Davide Marenduzzo, referring to the representation of DNA as beads and springs. In this model, 3000 base pairs are each grouped into spheres, some of which act as promoter-like transcription units (TUs), which represent genes to be transcribed, or as transcription factor complexes (TFs). University of Edinburgh researchers: Senior author Davide Marenduzzo (left) and first author Chris Brackley (right). (Courtesy: Davide Marenduzzo)
University of Edinburgh researchers: Senior author Davide Marenduzzo (left) and first author Chris Brackley (right). (Courtesy: Davide Marenduzzo)
 University of Edinburgh researchers: Senior author Davide Marenduzzo (left) and first author Chris Brackley (right). (Courtesy: Davide Marenduzzo)
University of Edinburgh researchers: Senior author Davide Marenduzzo (left) and first author Chris Brackley (right). (Courtesy: Davide Marenduzzo)“We spent some time to develop the force field in the past, starting from the work where we uncovered the bridging-induced attraction,” Marenduzzo tells Physics World, referencing a core component of the model. The multivalent interaction between TUs and TFs leads to their clustering and strongly affects the patterns of observed transcription. “The link comes from a surprisingly simple assumption…as transcriptional activity is measured computationally as the fraction of time a [transcription] factor spends close to a transcription unit,” he explains.
Firstly, the researchers looked at the behaviour of fragments comprising 1000 beads with randomly distributed TUs, interspersed by TFs. TUs that lie close together will cluster, which increases transcription locally but also leads to negative correlations of TUs that are further apart. In the case of a saturating number of TFs, the correlations remain local because there are enough TFs to bind all TUs without engaging in multivalent interactions. If, however, there are fewer TFs than TUs – as is the case in biological systems – the competition for TFs produces a well-linked, “small-world” network.
Within this framework, the researchers qualitatively recovered several characteristics of gene expression, such as short transcription bursts or a high variability between cells. Furthermore, they observed a rewiring of the network following the mutation of TUs or the introduction of fixed DNA loops.
Going beyond this so-called “toy system”, the authors succeeded in modelling entire human chromosomes based on experimental locations of the TUs. Marenduzzo reports that he was “very pleased we could get a significant agreement with transcriptional activity in human chromosome 14: while the correlation is not that large, what is good there is that there is no fitting [of the model parameters to match experimental data] in the model.”
Encouraged by these results, the team intends to “take these ideas forward by using much more sophisticated models…to build a compendium of 3D structure of all human genes and predict their transcriptional activities”.
The research groups of Marenduzzo and co-author Nick Gilbert have been awarded a Wellcome Trust grant to continue this collaborative work.
from physicsworld.com 20/10/2021
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου