Automated radiotherapy planning: a deep transfer learning approach
10 Jan 2022
Another challenging cancer site – another difficult radiation treatment to plan. Scientists at Duke University Medical Center and UNC Charlotte have developed a deep transfer learning model that automates radiotherapy planning for some of these tricky-to-plan cancers. They published their methods in Physics in Medicine & Biology.
Why transfer learning?
Wentao Wang, a medical physics resident at Duke University Medical Center, was a PhD student when much of this research was performed, under the guidance of Jackie Wu. Wang’s deep transfer learning model automatically creates intensity-modulated radiation therapy (IMRT) plans for adrenal stereotactic body radiation therapy (SBRT) cases using user-provided contours and dose constraints.
To avoid having to collect large amounts of high-quality data – something that’s not often available for treatment sites like the adrenal glands – before developing a model, Wang turned to transfer learning. Transfer learning decreases the number of cases and time needed to train a deep learning model by taking knowledge from a model that was trained on a large dataset and applying it to a different, yet related, dataset.
The deep transfer learning model was first trained on pancreas treatment plans. It then applies what it learned to the adrenal cancer cases. Both pancreatic and adrenal cancers must be planned carefully, sparing gastrointestinal organs that are sensitive to radiation. And because pancreatic and adrenal cancers are treated using different beam angles, beam settings and dose constraints, any deep transfer learning model must consider dose prescription differences and learned beam-to-beam interactions.
Wang uses two convolutional neural networks (CNNs) to directly generate fluence maps from patient geometry. The first CNN predicts the dose for every IMRT beam. The resulting beam dose volume is projected onto a 2D dose map that’s then used as the input for the second CNN. This second network predicts the fluence map for every IMRT beam, bypassing inverse optimization, a process that can lengthen manual planning times by hours or more. The fluence map can then be imported into the treatment planning system for multileaf collimator sequencing and final dose calculation.
“This study demonstrated the feasibility of using transfer learning to train deep learning models to create IMRT plans for adrenal SBRT,” Wang says. “It demonstrates that powerful deep learning tools such as transfer learning enable highly efficient learning with sparsely available clinical data.”
Evaluating the deep transfer learning model
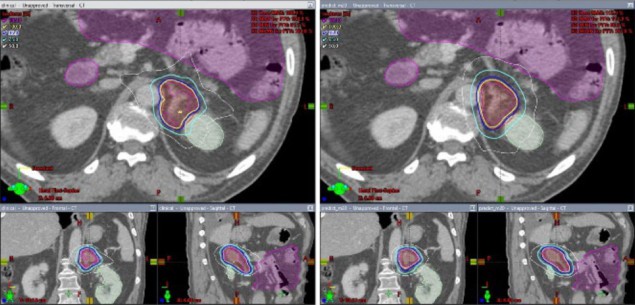
Wang and the rest of the team used high-quality IMRT plans to train the deep transfer learning model. Their base framework included 100 pancreas cases. Training, validation and testing of the transfer learning model was performed using 45 adrenal plans. Contours were drawn by dosimetrists and physicians. Final, optimized plans were made using the Eclipse treatment planning system, but the deep learning models, which were written in Python, can interface with any commercial treatment planning system.
The scientists’ deep transfer learning model generated plans in less than one minute. The team evaluated each plan by comparing dosimetric endpoints from plans created with the model to those from manually generated clinical plans. Results showed that the deep transfer learning plans produced plan quality scores approaching 80–90% of those from the clinical plans.
One disadvantage of the approach is that the deep transfer learning model was designed for IMRT cases with fixed gantry angles, while clinical plans at Duke often use a radiation therapy technique called volumetric modulated arc therapy (VMAT). In contrast to IMRT, VMAT delivers radiation dose continuously as the treatment machine rotates.
“For some challenging cases, the predicted plan could have slightly inferior plan quality compared to manually generated plans,” Wang says.
Yang Sheng, a medical physicist at Duke University Medical Center and another author on the study, notes that the deep transfer learning model can be a guide for dosimetrists and help them obtain optimal plans more quickly.
“With the power of deep learning, we are capable of processing dose image data of much higher capacity than dose-volume histograms, which allows us to predict a machine delivery parameter, aka the fluence map,” Sheng explains.READ MORE

“We can still manually tune the deep learning plans with inverse optimization starting from the deep learning plans,” Wang adds. “In the future, we expect to improve the model performance so that the deep learning plans would be able to replace manual planning.”
The scientists emphasize that their approach isn’t meant to replace dosimetrists and physicists, and final decision-making rests with the care team. This application of transfer learning could be used to adapt the model at institutions with different dose preferences, planning styles and more, they say. First, though, they are analysing their model to better understand its optimal configurations and limitations.
Catherine Steffel is a science writer and medical physicist based in Madison, Wisconsin. Catherine was previously a PhD student contributor to Physics World.
from physicsworld.com 14/1/2022
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου