Quantum batteries harvest energy from light
09 Apr 2022 Pradeep Niroula
Your night-mode photos might get a lot crisper thanks to a new device that exploits quantum mechanics to absorb photons more efficiently. Known as a quantum battery, the device stores the energy of the absorbed photons and can be charged simply by shining light on it — great news not just for low-light iPhone photography, but also for solar panels, which could use similar technology to capture the Sun’s energy a lot faster.
In a normal medium for storing energy, like a car battery, the time to charge the battery increases in proportion to the battery’s size. Quantum mechanical effects, however, make it possible to create systems with an energy absorption capacity that increases drastically as they get bigger. This is known as superabsorption, and it enables researchers to create a battery that, somewhat counter-intuitively, charges faster as its size increases.
Capturing light between mirrors
In the latest study, which is described in Science Advances, James Quach and colleagues at the University of Adelaide, Australia; the Politecnico di Milano and the National Research Center (CNR), Italy; and the Universities of St Andrews, Sheffield and Heriot-Watt in the UK created a quantum battery from molecules of an organic dye, trademarked Lumogen-F Orange. Dye molecules of this type can be modelled as an effective two-level system in which the molecule is most likely to be in one of two states: the ground state with minimal energy, or an excited state with higher energy. If laser light is fired at it at the right wavelength, it may absorb a photon and jump to the excited state.
To ensure that these dye molecules absorb photons efficiently, the researchers suspended them in a matrix of a non-reactive polymer and placed them in a cavity consisting of two mirrors. Because everyday mirrors (which are simply sheets of glass coated with reflective metal) are not reflective enough to keep the photons trapped in the cavity, the team used alternating layers of dielectric materials to create a device known as a distributed Bragg reflector.
When a laser beam shines into this highly reflective cavity, the dye molecules absorb photons and jump to the excited state. The amount of energy they absorb can be estimated by sending in a probe beam and monitoring how much of it gets reflected: once excited, molecules cannot absorb any more photons, so they reflect. Therefore, measuring the intensity of reflected light tells you how many dye molecules were excited, and how much energy was absorbed by the dye-battery.
The right concentration
To measure the battery’s charging rates, the group had to develop ultrafast measurement techniques to charge and probe the cavity in rapid succession, at time scales as short as 10 -14 seconds. The researchers also measured the absorption properties of different concentrations of dye while keeping the charging power constant. Remarkably, the time required to charge the cavity went down as the dye concentration went up, meaning that it took less time to charge N molecules in one microcavity than it would to charge N microcavities containing one molecule each. This phenomenon – a signature of superabsorption – occurs because the shared quantum entanglement between dye molecules lets them trap photons better than molecules on their own.
Given this result, one might wonder whether it would be possible to build batteries that charge instantaneously by increasing dye concentrations to some arbitrarily high level. Unfortunately, the way the molecules interact with each other and with light changes at very high concentrations, pushing the battery away from superabsorption. A further drawback is that because the dye molecules absorb energy faster, they also release it faster. While fast discharge may be desirable for some applications, like charging an electric vehicle, it is not good for a battery which should, ideally, store energy for a long time without dissipating it.
In this particular case, noise comes to the rescue. If the noise in the device is just right, the absorption-dissipation cycle can be disrupted as the excited dye molecules steer to something called a “dark mode” where photon emission is much suppressed.
Cavities in cameras
Although the dye molecules in this experiment are good at absorbing energy, and you can store a little bit of energy in a cavity, a useful battery would need to be able to extract the energy from the cavity before it could power, for instance, a mobile phone or a smart watch, or transport energy to a location where it can be stored long-term. To do this, the team would need to add additional components to the cavity that can transport the dye excitations outside, in the form of an electric current. “At the moment, the proof-of-principle devices we made are still quite small, and the charging occurs with light, so immediate applications would need to work with those constraints,” says Quach. Since superabsorption is a general quantum mechanical phenomenon, he adds, it may manifest in other systems as well.READ MORE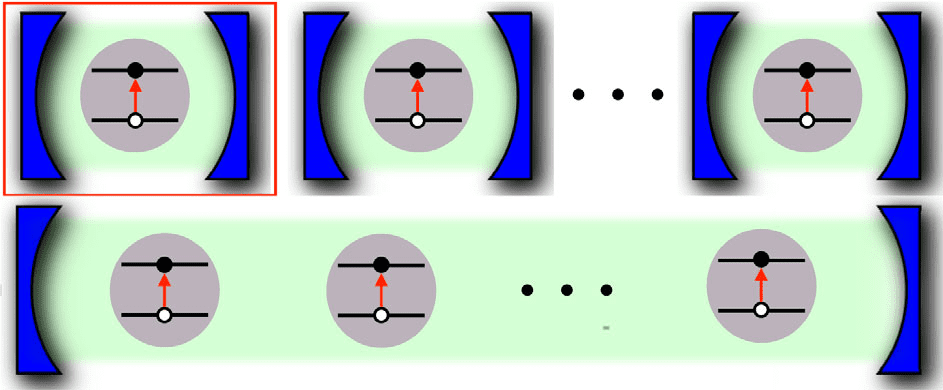

One near-term application of cavity-based quantum batteries would be to improve low-light energy capture in photovoltaic cells used in solar cells and cameras. However, substantial work remains to be done before we can reliably use superabsorption outside of a laboratory. For example, today’s solar cells and cameras can store energy of a wide range of wavelengths, but the quantum battery demonstrated in this experiment can only absorb light at a particular frequency. Quach says he and his colleagues are optimistic that they can scale up the system, and they are looking into ways of storing and transferring energy, with the goal of producing a device that can be easily integrated into existing technologies.

Pradeep Niroula is a PhD student contributor to Physics World. Pradeep is studying quantum error correction and complexity theory at the University of Maryland. Find out more about our student contributor networks
from physicsworld.com 12/4/2022

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου