High-spec open-source microscopy for all
10 Nov 2021
Taken from the November 2021 issue of Physics World. Members of the Institute of Physics can enjoy the full issue via the Physics World app.
Open-science hardware offers unprecedented technological access to researchers and enthusiasts all over the globe. Richard Bowman and Julian Stirling of the Bath Open Instrumentation Group describe the lessons learnt in developing a low-cost, laboratory-grade microscope

Six years ago, on a Friday afternoon, I (Richard Bowman) made a microscope focusing stage with the £400 3D printer in our lab. What started as a moment of idle curiosity quickly snowballed and pushed aside my “day job” research, to grow into so much more. Today, the OpenFlexure Project – developing a 3D-printed laboratory-grade motorized microscope to analyse samples and detect diseases – has a global community of users and developers that spans hobbyists, research scientists, entrepreneurs and clinical researchers.
All of them are supported by a core team spread around the globe – at the universities of Bath and Cambridge in the UK, along with the Tanzanian engineering company BTech (Bongo Tech & Research Labs, formerly STICLab) and the Ifakara Health Institute. We are currently working towards medical certification in Tanzania, and exploring commercialization options in various parts of the world. This growth has largely come about because of our commitment to openness and reproducibility – and the OpenFlexure project is just the beginning of what open-source hardware enables.
Complementary to open-source software, the open-source hardware movement aims to allow people all over the world to make, modify and share hardware for scientific use. Such technology could be particularly appealing for education and training purposes, where expensive professional equipment is particularly prohibitive. More essentially though, for researchers and medical professionals in the Global South, open-source hardware built and specified by themselves could be a game-changer.
Optical microscopes are an essential tool, both to detect disease in clinics, and for scientific research in general. But commercial high-performance microscopes are expensive – usually selling for tens of thousands of pounds – and are hard to maintain. Our most basic 3D-printed microscope, in contrast, can be built for as little as £15 (the cost of the printed plastic, a camera and some fastening hardware). Our top-end version – including a microscope objective and an embedded Raspberry Pi computer – would cost a couple of hundred pounds. If you visit openflexure.org, you can download, print and assemble the latest version of the OpenFlexure microscope. The fully automated microscope is highly customizable, with a number of options readily available for optics, camera and control, including motorized sample positioning and focus control.
Setting the stage
A key feature for any microscope is the ability to precisely position samples and probes – so it must have a precise and stable translation stage to focus the microscope, and move to the right area of the sample. This is a ubiquitous challenge when designing apparatus, and is one reason why research microscopes cost so much. Indeed, machining a fine translation stage that doesn’t wobble or stick requires smooth surfaces, hard materials and precise dimensions.
We found that simply 3D printing the stage designs used in most microscopes results in poor performance. That’s because printed plastic is soft, the surface finish is usually rough, and the printed object often doesn’t match its nominal dimensions exactly. Instead, our design exploits the flexibility of the plastic by using a deformable mechanism to move the sample. The “flexure hinges” that give the project its name are used in many precision instruments, but are usually made of metal and have a limited range of motion. The greater deformability of plastic, and the ability of 3D printers to create intricate shapes, mean our flexure stage can move a relatively long way: 12 × 12 × 4 mm.
The initial publication of the microscope in 2016 focused on its mechanics (Review of Scientific Instruments 87 025104). At that time, there were a number of low-cost and/or open-source microscope projects that provided a good optical solution, but there were far fewer easily manufactured solutions for the mechanics of such a microscope. We characterized our microscope’s drift over time; how repeatable the stage was when moved by stepper motors; and how linear the motion was. By all those metrics, our microscope compared well to others costing orders of magnitude more. Open and sustainable 3D rendering of the OpenFlexure microscope using an all open source toolchain (OpenSCAD and Blender). (CC-BY 4.0 Joel Collins)
Open and sustainable 3D rendering of the OpenFlexure microscope using an all open source toolchain (OpenSCAD and Blender). (CC-BY 4.0 Joel Collins)
 Open and sustainable 3D rendering of the OpenFlexure microscope using an all open source toolchain (OpenSCAD and Blender). (CC-BY 4.0 Joel Collins)
Open and sustainable 3D rendering of the OpenFlexure microscope using an all open source toolchain (OpenSCAD and Blender). (CC-BY 4.0 Joel Collins)By the time we’d completed this work, the OpenFlexure microscope was already starting to be noticed by others. A team in Cambridge picked up the OpenFlexure design and turned it into OpenScope – a programmable open-source microscope. Also, the ability to do low-cost timelapse imaging meant the microscope was the perfect base for the first generation of water-testing technology used by WaterScope – a company Richard co-founded in 2015 to provide low-cost methods for testing water quality in the Global South.
Now that we had active users, they started requesting features and improvements. In particular, we improved our optics options, which used conventional microscope objectives in addition to the inverted webcam lens that enabled most of the early work. The OpenFlexure microscope was then a capable lab prototype, able to take good quality images and even move the stage automatically. However, it was very much a lab prototype. The interface involved obscure keyboard commands with little documentation; there were loose wires and circuit boards; and the whole thing was usually packed inside a shoebox padded out with blue kitchen roll.
Building equitable partnerships
In 2018 we were funded by the UK’s Engineering and Physical Sciences Research Council and the National Institute for Health Research to develop and evaluate the microscope for malaria diagnostics – a project run in collaboration with Ifakara Health Institute and BTech in Tanzania. A central theme of this project is local manufacturing, rather than shipping microscopes from the UK to Tanzania, so that the microscopes used at the Institute can be built a couple of hours’ drive away, at BTech.
We believe this local manufacturing is crucial, as the World Health Organization estimates that nearly 70% of donated medical equipment in sub-Saharan Africa is out of service or not in use usually because authorized service engineers, proprietary consumables and spare parts are not available locally (Med. Biol. Eng. Comput. 49 719). Our approach means BTech can provide this missing infrastructure, thereby making a sustainable impact as well as creating skilled jobs. Ultimately, releasing the hardware under an open-source licence is a way to share ownership and control of the project, which we feel is key to building equitable partnerships between groups with very different economic contexts.
Working in collaboration, we improved the design to make it more acceptable in a hospital lab – for example, adding a better interface and enclosing the electronics. Together with BTech, we made it easier to manufacture too, adding 3D-printed tools for tricky steps, simplifying the bill of materials, and taking more care over the specification of the components. In particular, our initial prototype was very hard to reproduce as it required specific components that are expensive to order or re-order and are typically only found in well-funded labs. The current version of our microscope (Biomedical Optics Express 11 2447) is a capable lab microscope with options for high-resolution bright field, fluorescence and reflection imaging. Educational value Pathologist Daniel Rosen, at the University of Houston, using the OpenFlexure microscope for teaching. (Courtesy: Daniel Rosen)
Educational value Pathologist Daniel Rosen, at the University of Houston, using the OpenFlexure microscope for teaching. (Courtesy: Daniel Rosen)
 Educational value Pathologist Daniel Rosen, at the University of Houston, using the OpenFlexure microscope for teaching. (Courtesy: Daniel Rosen)
Educational value Pathologist Daniel Rosen, at the University of Houston, using the OpenFlexure microscope for teaching. (Courtesy: Daniel Rosen)Over the three and a half years that our malaria project has run so far, we have collected terabytes of images of blood smears, to evaluate the microscope and train machine-learning algorithms to understand the images. We have also identified and fixed many more issues that only came to light when we had multiple microscopes running long-term in realistic scenarios, in the field. When we scan a blood smear by taking a grid of images spread over the sample, for example, those images can now be evaluated on the fly, so that we can repeat any measurements that are out of focus. This approach of making the instrument smarter, so it can self-correct and become more reliable as well as more capable, is a key theme in the OpenFlexure project.
Open-science test drive
In tandem with our funded research focused on malaria, we have worked to build up a community around the microscope. Most project communication was initially done on the software-development platform GitLab. While GitLab is still where all our designs are managed, last year we set up an online forum that opened up the project to many more people. The forum is a much easier place for users and contributors to engage with the project without having to navigate the complicated, software-focused interface of GitLab.
Our community now includes scientists and engineers from all over the globe – from both physical and life science backgrounds – as well as hobbyists, teachers, members of community groups, and even companies using parts of the hardware or software in their own products. Our best estimate is that there are hundreds of microscopes that have been manufactured “in the wild” without our direct involvement. That means the OpenFlexure project is a useful testbed for many aspects of how to create and share a piece of open scientific hardware. Build your own An exploded render of the OpenFlexure microscope showing all components is part of the build instructions. (CC-BY 4.0 Joel Collins)
Build your own An exploded render of the OpenFlexure microscope showing all components is part of the build instructions. (CC-BY 4.0 Joel Collins)
 Build your own An exploded render of the OpenFlexure microscope showing all components is part of the build instructions. (CC-BY 4.0 Joel Collins)
Build your own An exploded render of the OpenFlexure microscope showing all components is part of the build instructions. (CC-BY 4.0 Joel Collins)The single most important thing we have learned about how to allow a piece of lab apparatus to be replicated exactly is the importance of good documentation. Our build instructions have gone through numerous complete rewrites and lots of minor updates – and there is still a long way to go. It’s not uncommon for replications of scientific experiments, even ones that intend to be fully open, to rely on direct communication between research teams. While this is less work for both teams the first time a project is replicated, it doesn’t scale well. Relying on word of mouth also means that crucial know-how never gets written down, which means the scientific record isn’t enough to reproduce an experiment once the researchers involved have moved on.
The growth of the OpenFlexure community has forced us to work in ways that scale better. Well-honed instructions are a good starting point, but moving all the questions and answers about the project from spoken or e-mailed conversations into an open, searchable forum means that not only the core instructions, but also a great deal of associated know-how is now available for others to learn from. It’s great to see more and more researchers adopting a similar approach, and we try very hard to make sure that what we’ve learned from this project is made available so that others can learn from our experience.
On the open record
One aspect of sharing a project openly that’s not often talked about is the emotional journey of opening up. The decision to release a project openly might seem simple, but there are many worries that often stop academics from fully sharing their work. Many scientists, for example, feel unable to share details before their work is published, in case they are “scooped”. We too did not initially share the first version of the OpenFlexure microscope online – only doing so when the preprint of our first paper was uploaded to arXiv – but we have since moved to sharing projects much earlier. Doing so eliminates worries about who we share ideas with, and more fully realizes the benefits of open working (The Design Journal 10.1080/14606925.2020.1859168). Sharing designs as they evolve actually makes us less concerned about others “stealing” our work, as there is a verifiable, public record of who did what in our repositories. This makes it much harder for someone to claim the work as their own, than if we kept all of our work a secret until we’re ready to publish.
One aspect of sharing a project openly that’s not often talked about is the emotional journey of opening up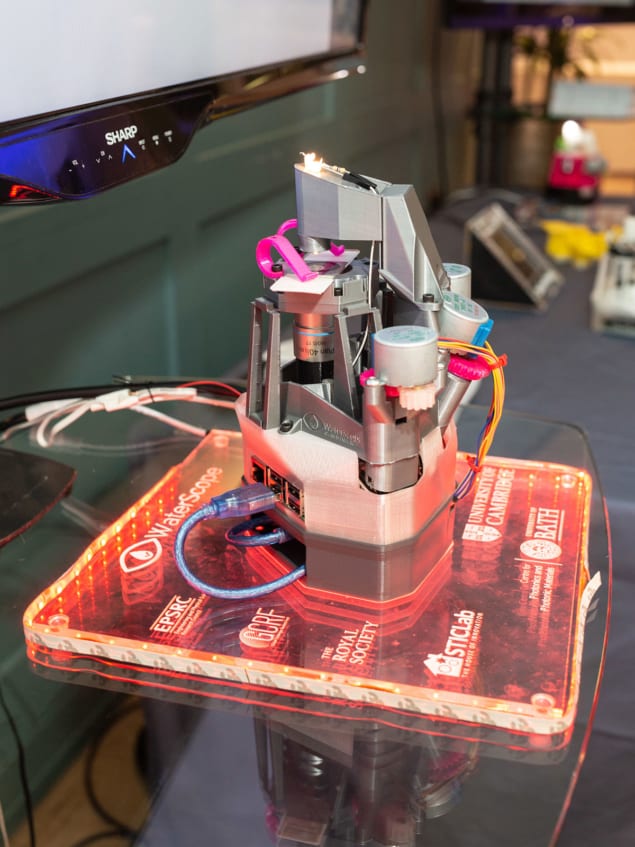 The OpenFlexure microscope adapted for the WaterScope project. (Courtesy: University of Bath)
The OpenFlexure microscope adapted for the WaterScope project. (Courtesy: University of Bath)
 The OpenFlexure microscope adapted for the WaterScope project. (Courtesy: University of Bath)
The OpenFlexure microscope adapted for the WaterScope project. (Courtesy: University of Bath)The deeper worry, however, is usually that releasing the project openly will diminish the creators’ ownership and control of it; meaning they don’t receive full credit for the work – be it financial, or through authorship and citation of published articles. This is a reasonable concern, but our experience is that the benefits far outweigh the downsides. By and large, people who make use of open designs are happy to give credit where it’s due. After all, citing the design properly is a small effort to repay something useful, and the scientific community is increasingly paying attention to good practice around open science.
While there may be people and projects who have not credited their use of our work properly, this is more than made up for by the large network of collaborators who do recognize its value. That network is far bigger than it would be if we had taken a less open approach, and indeed it is much larger than we could support if we had to deal with requests individually rather than through open, reusable platforms like the forum.
Experimental ecosystem
As scientific instruments go, the OpenFlexure microscope is more of a workhorse tool than a state-of-the-art microscope existing in only a few labs. However, it has been extended to do super-resolution and phase imaging, and can be connected to another open-source toolbox – the UC2 modular optics system – for even more customizable use. Valerian Sanga adjusting the illumination of an OpenFlexure microscope. (CC-BY 4.0 Gathering for Open Science Hardware)
Valerian Sanga adjusting the illumination of an OpenFlexure microscope. (CC-BY 4.0 Gathering for Open Science Hardware)
 Valerian Sanga adjusting the illumination of an OpenFlexure microscope. (CC-BY 4.0 Gathering for Open Science Hardware)
Valerian Sanga adjusting the illumination of an OpenFlexure microscope. (CC-BY 4.0 Gathering for Open Science Hardware)One of the reasons it has been extended so frequently is its ease of replication: it is simple and inexpensive enough for other labs to replicate on a whim, without feeling the need to justify their efforts with a new publication or apply for a research grant to pay for it. A big part of our vision for the project is that it can support an ecosystem of experimental techniques that can be fully replicated at moderate cost. Our hope is that this will lead to experimental science becoming more transparent, being repeated more often, and ultimately improving the quality and trustworthiness of our experiments.
The tools and working practices we develop to support the sharing of instrument designs need active, frequently replicated projects like ours for their development, but are intended to scale well to more expensive and specialist projects. This is reflected in CERN’s White Rabbit Project – a multi-laboratory, multi-company collaboration to develop new technology for control and data-acquisition systems that covers hardware, gateware and software; or the mesoSPIM initiative, which focuses on open-source light-sheet microscopes (Nature Methods 16 1105).
Support and funding in the long run
Looking to the future, one of the biggest obstacles to achieving a long-term, sustainable impact with a project such as ours is supporting the team over a number of years. We are fortunate to have been funded for five years through the Global Challenges Research Fund (GCRF) – part of the UK’s aid budget that funds partnerships between UK scientists and their counterparts in low and middle-income countries. Building the trust that is needed for a productive, equitable partnership takes a long time, as does learning to work around the many differences in culture and working practices between different countries and institutions.READ MORE

The project so far has been a great success, but recent cuts to the UK aid budget mean that future funding for our work, as well as hundreds of other projects, is now scarce and the phase 2 funding for our pan-African network was cancelled earlier this year. The GCRF represents a significant investment from the UK, and if we are serious about achieving a meaningful impact with this kind of work we must make sure that we have a plan in place to support its translation out of academia. In the UK, this support often comes from companies that will commercialize the work, supported by government grants.
If we are serious about achieving a meaningful impact with this kind of work we must make sure that we have a plan in place to support its translation out of academia
In the spirit of “sustainable development”, we want to avoid leaving our African partners dependent on a UK company, as this would undermine many of the key benefits of an open, distributed project. However, start-up capital and support for small businesses is much harder to come by in countries with fewer economic resources. Transferring work from academia into charitable organizations is also harder, and much less well supported, than simply patenting and licensing technologies in a way that entrenches the inequalities already present in the world’s economic system.
On an individual level, this project could not have succeeded without postdoctoral researchers on short-term contracts who have devoted several years to building relationships, sharing knowledge and documenting projects to get ready for real-world use – often at the expense of chasing prestigious publications that would further their career. The recent aid cuts, and the consequent slashing of the GCRF programme, means that these incredibly valuable and committed people will now have to find jobs elsewhere. This comes at a huge personal cost to the people involved, but it also means that their skills, knowledge and partnerships are lost.
There’s a great deal of anger from the GCRF research community that has flourished in recent years, and problems will continue even if the UK does reinstate aid funding to meet its legal aid budget commitment of 0.7% of gross national income. We are pursuing other ways that our project and others like it can continue to make progress, for example by creating a foundation that can help open projects move towards medical certification, and are keen to collaborate with others to bring this about. We believe that open-source hardware truly has the potential to revolutionize the way we do science – from building instruments and developing educational tools, to launching clinical applications and growing global partnerships.
from physicsworld.com 11/11/2021
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου