Microscopy technique inspired by Kelvin reveals long-sought ‘sigma-hole’
24 Nov 2021
Scientists have long suspected that bonds between certain negatively-charged halogen atoms are made possible by regions of positive charge called sigma-holes, but they lacked experimental proof. Now researchers in Czechia have used a method known as Kelvin probe force microscopy to image these sub-atomic structures directly – an achievement they claim will lead to a better understanding of molecular crystals and the folding of biomolecules, among other phenomena.
The stability of certain molecular crystals had long been a puzzle to scientists, since the crystals contain pairs of negatively-charged atoms that would ordinarily be expected to repel one another. These pairs consist of either two atoms from the halogen group of elements (such as bromine), or one halogen atom and another electronegative atom such as oxygen or nitrogen.
Several groups have proposed that halogen bonds come about through an anisotropy in the atoms’ charge distribution. In other words, rather than having a spatially uniform negative charge, they consist of a belt of negative charge topped off by a positively-charged crown – the sigma-hole. However, while quantum-mechanical simulations and observations of crystal structures provided indirect support for this idea, no-one to date was able to image the anisotropy directly.
Kelvin probe force microscopy
A group headed by Pavel Jelínek of the Czech Academy of Sciences in Prague and Palacký University in Olomouc has now achieved this feat by exploiting Kelvin probe force microscopy. Based on work originally carried out by William Thomson (Lord Kelvin) at the end of the nineteenth century, and adapted more recently to image intramolecular charge distribution, the technique involves suspending a tiny cantilever over a sample and electronically connecting the two so that they form a capacitor. The next step is to set the cantilever vibrating and record how its frequency of vibration shifts as it is brought close to the sample. This shift is measured over a range of voltages and the resulting distribution plotted as a “Kelvin parabola” with a peak at a particular value. This value represents the difference between the work function – a macroscopic quantity describing how much energy is needed to remove an electron from a surface – of the cantilever tip and that of the sample.
Scientists had previously used a Kelvin probe force microscope with a tip comprising a single atom to map the atomic-scale local variation in charge density by measuring shifts in the parabola’s peak. Jelínek and colleagues have now refined the theoretical understanding of this technique and optimized the experimental procedure. This allowed them to enhance the sensitivity of the electrostatic interaction between probe and sample, and thereby push the technique’s resolution beyond the atomic scale.
The researchers carried out their experiment by depositing molecules of a bromine-containing compound (tetrakis(4-bromophenyl) methane) on a silver surface inside an ultra-high vacuum at cryogenic temperatures. Each molecule had a tripod-like shape, with the bromine atom uppermost and so easily probed. At the cantilever tip the researchers placed a xenon atom, which has a uniform charge distribution and therefore avoids confounding signals. By moving the probe through a grid of points above the bromine atom and plotting a separate Kelvin parabola at each point, they were able to map regions of higher and lower electron density within the halogen atom.READ MORE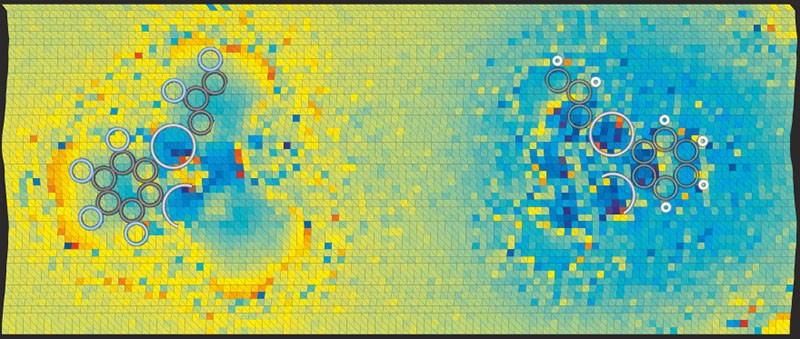

In doing so, the researchers found that the bromine atoms do indeed contain a sigma-hole surrounded by a more negative bulk. They backed up their result with simulations based on density functional theory, and also carried out analogous experimental measurements involving the same molecules but with fluorine replacing the bromine. Although fluorine is also a halogen, it attracts electrons so strongly when forming chemical bonds (it is highly electronegative) that a sigma-hole cannot develop. The team’s measurements revealed, as expected, that the fluorine atoms have a uniform negative charge distribution.
Direct imaging of anisotropic atomic charge
According to Jelínek, the findings not only confirm the existence of the sigma-hole and the concept of halogen bonds but also constitute the first direct imaging of anisotropic atomic charge. “The resolution of the sigma-hole opens up a new way to characterize the electron density of single atoms,” he says. “We can now think about measuring the electron cloud’s response to an external field.”
Among the systems that could be probed in more detail, he adds, are atomic defects in two-dimensional materials. Kelvin probe force microscopy, he says, could be used to establish whether a defect is positively or negatively charged and whether the charge distribution is asymmetric or not.
The research is published in Science.
Edwin Cartlidge is a science writer based in Rome
from physicsworld.com 24/11/2021

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου