Caution required: anesthesia with supplemental oxygen can impact proton therapy
22 Jan 2024
Protecting the brain A preclinical study has demonstrated the need to optimize anaesthesia and supplemental oxygen protocols when using conventional or FLASH proton therapy to treat brain tumours. (Courtesy: iStock/herjua)
Many children who receive proton therapy for brain tumours do so under general anaesthesia or sedation, an approach that guarantees reproducible positioning and targeted delivery of radiation. They may also receive supplemental oxygen, which reduces the risk of adverse effects related to the use of general anaesthesia.
Yet, until recently there were no studies assessing the impact of this supplemental oxygen – which changes oxygen saturation in the blood during irradiation – in conventional or FLASH (ultrahigh dose rate) proton therapy.
Understanding the impact of supplemental oxygen is critical, says Yolanda Prezado, CNRS research director and group leader of the new approaches in radiotherapy (NARA) team based at Institut Curie. While FLASH radiation therapy has been reported in preclinical studies to reduce complications, most studies have been performed with electron – rather than proton – beams. And the mechanisms underlying radiation-induced cognitive changes are poorly understood.
“Cognitive deficits have been reported in some survivors of paediatric brain tumours,” says Prezado. “We thought it was a good idea to try to understand the normal brain response [in rats] in proton therapy beams. And even more important motivation was that there was never a systematic study of the impact of anaesthesia in patients. What we saw in our study is that this could induce complications.”
Prezado’s team collaborated with radiation oncologists and anaesthesiologists to observe the effects of supplemental oxygen in rats. In the study, 36 rats were divided into “with glioblastoma” and “without glioblastoma”, and anaesthesia (“no O2”) and anaesthesia with supplemental oxygen (“with O2”) groups. The animals received a single unilateral dose of proton radiation (either 25 or 15 Gy, a dose similar to that used in previous electron FLASH studies) at either a conventional dose rate (4 Gy/s) or a FLASH dose rate (257 Gy/s) using a 226 MeV clinical proton beam. Film dosimetry was used to verify irradiation conditions.
The researchers, reporting their results in Communications Medicine, found that supplemental oxygen had an adverse impact on both the function and structure of rats’ normal brain tissue following both FLASH and conventional proton therapy. Rats receiving FLASH proton therapy with supplemental oxygen had the highest level of brain injury observed on MRI (using a 7 T preclinical magnet with Gd-DOTA contrast), histology and behavioural tests. Animals treated with FLASH without supplemental oxygen had the lowest degree of brain injury. Despite reduced side effects in this group, brain tissue damage was still observed following a therapeutic dose for gliomas in rats (25 Gy).
As reported in other studies, FLASH proton therapy resulted in memory sparing compared with conventional proton irradiation. But incorporating supplemental oxygen had detrimental effects on recognition memory after both conventional and FLASH proton therapy. These effects persisted six months after irradiation. Such observations, the researchers say, are consistent with previously published data in electron FLASH therapy – one study showed that supplemental oxygen suppressed the protective FLASH effect on cognitive function two months following irradiation.
Supplemental oxygen and combination therapies
The research team also identified a previously unobserved relationship between oxygen saturation, dose rate and radiation-induced immune response. Generally, high concentrations of supplemental oxygen prevented immune cell infiltration into the tumour, but the tumour infiltration of immune cells following FLASH proton therapy was less impacted than for conventional proton therapy.
This finding, the researchers say, demonstrates that oxygen supplementation is less influential in FLASH proton therapy than in conventional proton therapy and suggests that radiation-induced immune regulatory pathways are susceptible to proton beam dose rate.
A possible alternate explanation for some of the researchers’ results could be lipid peroxidation of phospholipids, which has been shown to alter cell signalling, dysfunction or death, and may be involved in brain aging. Though lipid peroxidation (an increased probability of biomolecular recombination of fatty acids that have lost a hydrogen ion from the OH radical) has not been demonstrated following FLASH, the researchers suggest that a study be performed.READ MORE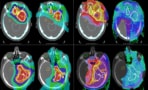

Limitations of the study include a small sample size and that no oxidative parameters were monitored experimentally. Still, the researchers say that they hope their research prompts medical doctors to examine current anaesthesia protocols and revise them to reduce the neurocognitive side effects of both conventional and FLASH proton therapy. The potential impact of supplemental oxygen on immune cell infiltration with combination therapies, such as radioimmunotherapy, should also be considered.
“What I think it is relevant to do is a retrospective evaluation of paediatric patients treated with radiotherapy,” Prezado says. “This [study] was a word of caution to medical doctors to say, you need to optimize your protocols…The main point is to raise some concerns, to raise the point about the potential effects of anaesthesia and supplemental oxygen. This has been discussed for other reasons in the medical community…but it raised a question mark on, we need to think about anaesthesia for the patients when they are being evaluated…The community was saying FLASH can be wonderful for paediatric patients, but seeing the results, I think further evaluations are still needed.”
Catherine Steffel is a science writer based in Madison, Wisconsin. Catherine was previously a PhD student contributor to Physics World.
FROM PHYSICSWORLD.COM 28/1/2024
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου