FLASH irradiation spares immune cells during proton therapy
30 Jan 2024 Tami Freeman
Modelling blood flow in the brain The spatiotemporal propagation of blood (violet) through the vessels (yellow) in the reconstructed brain model. Left to right: the distribution at the start (0.2 s), after 1.5 s and at equilibrium (longer then 7s). (Courtesy: CC BY 4.0/Phys. Med. Biol. 10.1088/1361-6560/ad144e)
Treating cancer with radiation can stimulate the body’s immune response and inhibit tumour growth, but it can also reduce the level of lymphocytes, the white blood cells associated with immune response, resulting in impaired tumor control and poor prognosis. The severity of this radiation-induced lymphopenia correlates with the dose delivered to circulating blood cells and lymphocytes. As such, minimizing dose to the heart, peripheral blood and lymphoid organs could help reduce this detrimental effect.
To investigate this theory further, Antje Galts and Abdelkhalek Hammi from TU Dortmund University explored whether FLASH radiotherapy – radiation delivered at ultrahigh dose rates – could reduce the level of immune cell depletion during proton therapy of brain cancer patients.
“The biological mechanism behind the observed FLASH sparing effect at high dose rates is not yet fully understood. However, one of the proposed theories is the immune hypothesis, which suggests that the instantaneous dose delivery of FLASH irradiation significantly reduces the depletion of circulating lymphocytes by minimizing exposure time,” Hammi explains. “In our study, we showed that a hypofractionated treatment and fast dose delivery spared immune cells by up to 27 times compared with a conventional fractionated proton pencil-beam scanning treatment plan.”
Galts and Hammi used a dosimetric blood flow model to simulate the dose to circulating lymphocytes during conventional and FLASH-based intensity-modulated proton therapy (IMPT) of a brain tumour. The dynamic beam delivery model simulates an IMPT fractionated treatment plan while considering the spatiotemporal variation of dose rate of each single proton pencil beam. Hammi notes that the model incorporates realistic delivery parameters from commercially available cyclotrons.
To accurately reflect blood circulation in the human brain, Galts and Hammi mapped blood vessels directly from brain MR angiography images. They used the resulting cerebrovascular model, which included 465 blood vessels and 8841 individual vessel branches, to simulate the circulation of immune cells within the blood stream.
The researchers created realistic IMPT treatment plans for a glioblastoma tumour, using four incident proton beams and clinically relevant delivery parameters. They then calculated the time-varying radiation fields that the circulating blood is exposed to during delivery of the proton therapy plans and the accumulated dose following treatment, reporting their findings in Physics in Medicine & Biology.
Glioblastoma is the most lethal form of brain cancer and treating it with radiotherapy can cause prolonged radiation-induced lymphopenia. “By modeling a cerebrovascular system during radiation delivery, we hope to gain deeper insights into how radiotherapy affects the immune response in these groups of patients, potentially leading to improved therapeutic strategies,” says Hammi.
Plan comparisons
Galts and Hammi examined four treatment scenarios: IMPT FLASH with a single 22.3 Gy fraction; hypofractionated FLASH using two 14.6 Gy and five 8 Gy fractions; and conventional IMPT using thirty-two 2 Gy fractions. For each treatment plan, they assessed the dosimetric impact on the circulating lymphocytes and estimated the resulting radiotoxicity.
Dose–volume histograms revealed that FLASH radiotherapy significantly reduced the proportion of irradiated cells compared with conventional dose rate IMPT. During the first treatment fraction, all three FLASH schemes irradiated around 1.52% of the circulating blood volume, while conventional IMPT irradiated 2.18%. Hypofractionated FLASH plans, delivered over two or five fractions, increased this irradiated volume to 3.01% and 7.35%, respectively, while conventional IMPT exposed 42.41% of peripheral blood to radiation.
Next, the researchers examined the level of circulating lymphocytes that received a dose of at least 7 cGy – a threshold that causes 2% depletion in lymphocyte population – during the entire treatment. After completing conventional IMPT, 25.65% of the circulating lymphocytes received a dose of at least 7 cGy. For single-, two- and five-fraction FLASH treatments, the volumes receiving more than this dose threshold were 1.21%, 2.30% and 5.14%, respectively.
The volumes of circulating lymphocytes receiving doses of more than 100 cGy, which causes 30% depletion, were 0.77%, 1.28% and 2.09% for single-, two- and five-fraction FLASH, respectively, and 0.10% during conventional IMPT.
Galts and Hammi also studied the response of CD4+ and CD8+ lymphocytes, which have different distributions in peripheral blood, to the various irradiation scenarios. For both lymphocyte types, cell killing after the first fraction was 0.66%, 0.62%, 0.32% and 0.08% for single-, two- and five-fraction FLASH, and conventional IMPT, respectively.READ MORE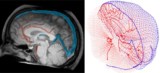

After the full treatment, the depletion in lymphocytes was 1.02% and 1.56% for two- and five-treatment fractions, respectively, and 2.14% for conventional IMPT. These findings demonstrate that FLASH proton therapy spares circulating immune cells during intracranial treatment, with single-fraction FLASH reducing the depletion rate by almost 70% compared with conventional IMPT.
Hammi tells Physics World that they are now expanding the model to include head-and-neck cancers. “Furthermore, we are exploring various FLASH delivery methods and their impact on the depletion of the immune system, with a particular focus on conformal FLASH treatment that’s based on passive, patient-specific energy modulation,” he explains. “This delivery model has the potential to spare more circulating lymphocytes compared with shoot-through FLASH delivery.”

Tami Freeman is an online editor for Physics World
FROM PHYSICSWORLD.COM 1/2/2024

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου