Multishelled fullerenes beat graphene at catalysing water splitting
05 Jul 2019 Henry R N B Enninful
Moving from flat to curved catalytic carbon supports could help reduce the amount and cost of platinum needed for hydrogen production in water splitting. In a new study published in Nature Energy, aimed at improving the efficiency of electrocatalytic processes, a team of scientists led by Li Song and Jun Jiang from the University of Science and Technology of China (USTC) used platinum decorated spherical onion-like carbon (OLC) catalysts with platinum atoms deposited on their outermost surface instead of flat graphene supports. This method reduced the amount of platinum atoms otherwise needed by about 75% while maintaining or improving the rate of the electrochemical hydrogen production process.
Catalysts and Energy
Energy is the fuel for growth and life. Despite centuries of over-reliance on fossil-based energy sources with devastating effects on the planet, more environmentally friendly and renewable options keep emerging as suitable alternatives.
Synthesizing spherical carbon supportsHydrogen generation by water splitting has long attracted attention due to its zero-emission advantage. Water splitting or electrolysis is a technique employed in decomposing water molecules into hydrogen and oxygen by the passage of an electric current. It requires excess energy to overcome the reaction’s activation barriers; hence, it requires catalysts to increase the efficiency of the chemical process.
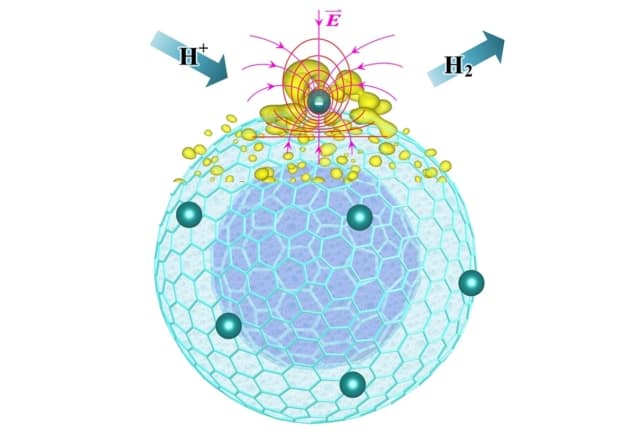
In this novel approach, the researchers created OLC supports by treating surface-oxidized nanodiamonds at various temperatures to deoxygenate them. This treatment transformed the nanodiamonds into OLCs at temperatures above 900 °C. They then dispersed platinum (Pt) atoms onto the spherical carbon supports by the atomic layer deposition method, which avoids clustering. Annealing the oxidized nanodiamonds at 1500 °C resulted in multishelled spherical graphene structures – that is, fullerene. These OLC particles have a diameter of about 5 nm and interlayer distances of 0.35
Ruthenium catalyst sets new efficiency record for water splitting
Reduced amount of platinum
These optimized OLC electrocatalysts utilize only 0.27 wt% Pt to achieve a comparable amount of hydrogen production to that of commercial Pt-carbon catalysts, which have 20 wt% Pt. Moreover, they improve the hydrogen production rate compared with graphene-supported catalysts with a similar Pt loading.
Complementary computer simulations suggest that the curved carbon structure with platinum atoms deposited on it leads to strong highly localized electric fields. Other researchers have reported similar field enhancements associated with a sharp tip – akin to the effect that makes lightning rods work – in the catalysis of CO2. They suggest the highly localized enhanced electric field makes the water-splitting reaction more efficient.

Henry R N B Enninful is a PhD student contributor to Physics World. Henry is investigating the characterization of complex nanoporous materials at the Felix Bloch Institute for Solid State Physics. Find out more about our student contributor networks
from physicsworld.com 11/7/2019
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου