Radiation can reverse heart rhythm disorders by reprogramming damaged cardiac cells
02 Nov 2021
Researchers at Washington University School of Medicine in St. Louis have found that radiation may reverse heart rhythm disorders, such as arrhythmias, long-term by reprogramming damaged heart cells.
“There are many ways to stop an arrhythmia. One is with catheters; another is with drugs. But no treatment previously has been shown to modulate how fast that [electrical] impulse travels,” says Stacey Rentschler, a physician scientist. “What we’ve seen is that radiation therapy can modulate how fast the impulse travels by changing the expression of genes and proteins in the heart.”
Rentschler is a member of the Center for Noninvasive Cardiac Radioablation, an interdisciplinary research team at Washington University School of Medicine that is working to treat life-threatening heart rhythm disorders safely and noninvasively. The team’s latest study, published in Nature Communications, begins to unravel how photon irradiation reduces ventricular tachycardia, a disorder caused by abnormal electrical signals in the heart’s lower chambers.
A puzzling result
Unlike catheter ablations, which are invasive and time-consuming, or drugs, which have side effects and are only moderately effective, radiation therapy noninvasively delivers ablative doses of radiation to specific regions of the body and could replicate the effects of catheter ablations. Catheter ablations destroy tissue quickly to create a fibrotic response that stops arrhythmias by preventing re-entry of electrical impulses into the ventricles.
The researchers found that a one-time delivery of 25 Gy of photon radiation to the whole hearts of mice – and to segments of ventricles containing scarring in humans – reduced the frequency of arrhythmias in only a few weeks.
And that puzzled them. They had presumed that radiation would prevent arrhythmias by creating fibrosis in areas of ventricular scarring, but their studies on human hearts showed that fibrosis alone wasn’t enough to explain the therapeutic effect.
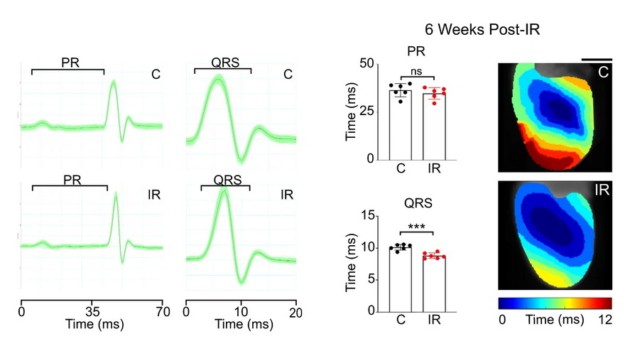
Then, they realized that anti-arrhythmic effects could be achieved through elevated levels of cardiac conduction proteins and improved conduction of electrical impulses in the heart. They confirmed this hypothesis by looking at the structural and physiologic changes to mouse hearts observed after irradiation.
The team’s next step was to identify which biological pathways are implicated in this electrophysiological reprogramming. They identified a reactivation of Notch signalling – known to play a critical part in human development and a role in the response of several tissue types to radiation – as a potential mechanism whereby radiation may reprogramme conduction.
Patients at Washington University School of Medicine in St. Louis receiving cardiac radioablation have experienced reductions in arrhythmias for two years and counting.
New avenues for cardiac radioablation
With this latest research, Rentschler and colleagues have embarked on a new line of radiobiologic inquiry.
“For the longest time, radiation oncologists avoided [irradiating] the heart to avoid cardiotoxicity,” explains first author David Zhang, a medical and PhD student in the Medical Scientist Training Program at Washington University School of Medicine in St. Louis. “What we’re starting to understand now is that because cardiomyocytes are not actively dividing, they don’t really undergo the same radiobiologic processes in response to double-stranded DNA breaks from radiation as actively-dividing cells.”
Zhang notes that while the single-arm, single-centre study design, the low number of patients enrolled and narrow patient selection are limitations of the study, this work demonstrates that clinical cardiac radiotherapy is associated with molecular and functional changes in electrophysiology.
“The Notch pathway is generally a strong regulator of epigenetics in the heart. And we now have a very strong suspicion that radiation is leading to many epigenetic changes in the heart that could cause or explain why these effects are persisting for longer periods of time,” says Zhang. “It’s really led us towards that epigenetic pathway to understand what this radiation-induced cardiac reprogramming is actually doing.”
Clifford Robinson, a radiation oncologist involved with the study, commented that treatment plans incorporate total cardiopulmonary motion measured from simulation 4D-CT scans and that motion margins are asymmetric. Target volume margins are symmetric. Results from additional preclinical studies may impact this protocol, Robinson said.READ MORE
In these studies, Robinson, radiation oncologist Julie Schwarz, and other members of the team are studying how dose impacts therapeutic effects: preclinical studies in mice suggest that a lower dose of 20 Gy may produce similar improvements in conduction as a 25 Gy dose, meaning that reactivation of the Notch pathway could be induced at lower levels of radiation and may be preferred for large treatment volumes.
“I think [this study] has opened up a new thought of how to treat arrhythmias that might be possible to, instead of destroying tissue, to rejuvenate unhealthy tissue…I think there’s a lot of excitement and a lot of interest in understanding the mechanisms even more so that we can make this therapy safer, more effective and potentially expand to other populations of patients, as well,” says Rentschler.
from physicsworld.com 2/11/2021
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου