A glassy solution to nuclear waste
22 Jun 2022
Taken from the June 2022 issue of Physics World. Members of the Institute of Physics can enjoy the full issue via the Physics World app.
Ancient glass is not just of interest to historians and archaeologists – it may also hold the key to understanding the durability of vitrified nuclear waste. Rachel Brazil investigates
The golden death mask of the pharaoh Tutankhamun is one of the most famous historical artefacts in the world. The shining visage of the young king dates back to around 1325 BCE and features blue strips that are sometimes described as lapis lazuli. Yet rather than being the semi-precious stone favoured in ancient Egypt, the striking decoration is in fact coloured glass.
A coveted and highly prized material deemed worthy of royalty, glass was once viewed on a par with gemstones, with examples of ancient glass going back even further than Tutankhamun. Indeed, samples excavated and analysed by archaeologists and scientists have enabled a better understanding of how and where glass production began. But surprisingly, ancient glass is also being studied by another group of scientists – those who are finding safe ways to store nuclear waste.
Next year the US will start to vitrify parts of its legacy nuclear waste currently housed in 177 underground storage tanks at the Hanford Site, a decommissioned facility in Washington state that produced plutonium for nuclear weapons during the Second World War and Cold War. But the idea to transform nuclear waste into glass, or vitrify it, was developed as far back as the 1970s, as a way to keep the radioactive elements locked away and prevent them from leaking out.
Nuclear waste is typically classified as being low, intermediate or high level, depending on its radioactivity. While some countries vitrify low and intermediate-level waste, the method is mostly used to immobilize high-level liquid waste, which contains fission products and transuranic elements with long half-lives that are generated in a reactor core. This type of waste requires active cooling and shielding because it is radioactive enough to significantly heat both itself and its surroundings.
Before the vitrification process, liquid waste is dried (or calcined) to form a powder. This is then incorporated into molten glass in huge smelters and poured into stainless steel canisters. Once the mixture has cooled and formed a solid glass, the containers are welded closed and readied for storage, which nowadays takes place in deep underground facilities. But the glass does not just provide a barrier, according to Clare Thorpe, a research fellow at the University of Sheffield, UK, who is studying the durability of vitrified nuclear waste. “It’s better than that. The waste becomes part of the glass.”
The glass does not just provide a barrier. It’s better than that. The waste becomes part of the glassClare Thorpe, University of Sheffield, UK
However, there have always been question marks over the long-term stability of these glasses. How, in other words, can we know if these materials will remain immobilized over thousands of years? To better understand these questions, nuclear-waste researchers are working with archaeologists, museum curators and geologists to identify glass analogues that might help us understand how vitrified nuclear waste will change with time.
Ingredient sweet spot
The most stable glasses are made from pure silicon dioxide (SiO2), but various additives – such as sodium carbonate (Na2CO3), boron trioxide (B2O3) and aluminium oxide (Al2O3) – are often incorporated to change the properties of the glass, such as viscosity and melting point. For example, borosilicate glass (containing B2O3) has a very low coefficient of thermal expansion, so does not crack under extreme temperatures. “The UK and other countries, including the US and France, have chosen to vitrify their waste in borosilicate glass before it’s stored,” explains Thorpe.
When elements such as those from additives or nuclear waste are included, they become part of the glass structure as either network formers or modifiers (figure 1). Network-forming ions act as a substitute for silicon, becoming an integral part of the highly cross-linked chemically bonded network (boron and aluminium do this for example). Meanwhile, modifiers interrupt the bonds between oxygen and the glass-forming elements by loosely bonding with the oxygen atoms and causing a “non-bridging” oxygen (sodium, potassium and calcium incorporate this way). The latter cause weaker overall bonding in the material, which can reduce the melting point, surface tension and viscosity of the glass overall.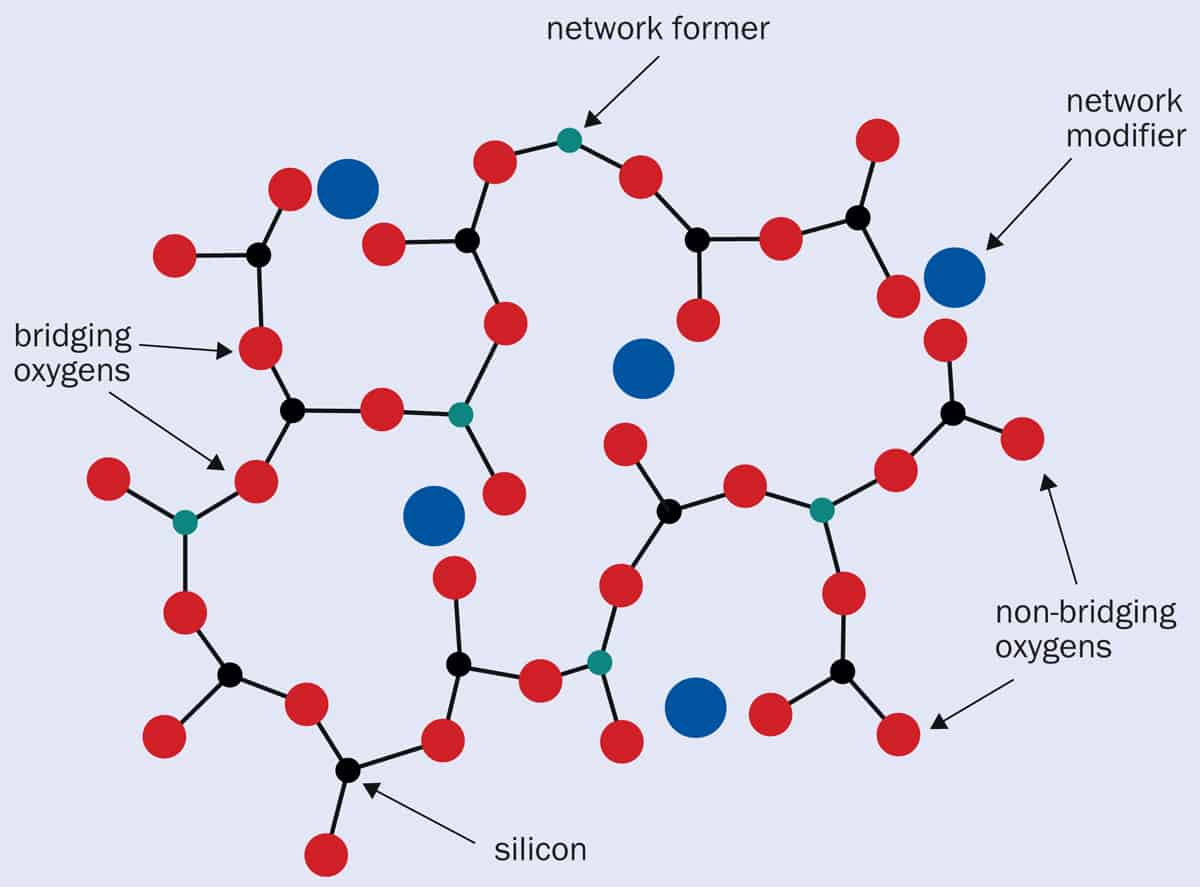 1 Formers and modifiers When an additive is incorporated into a glass mixture, the ions either become part of the highly cross-linked network, replacing the silicon (black dots) as network formers (green dots), or act as glass modifiers (blue dots) that loosely bond with the oxygen (red dots) and disrupt the glass-forming bonds, creating “non-bridging” oxygens. (Adapted from Clinical Applications of Biomaterials 10.1007/978-3-319-56059-5_2)
1 Formers and modifiers When an additive is incorporated into a glass mixture, the ions either become part of the highly cross-linked network, replacing the silicon (black dots) as network formers (green dots), or act as glass modifiers (blue dots) that loosely bond with the oxygen (red dots) and disrupt the glass-forming bonds, creating “non-bridging” oxygens. (Adapted from Clinical Applications of Biomaterials 10.1007/978-3-319-56059-5_2)
 1 Formers and modifiers When an additive is incorporated into a glass mixture, the ions either become part of the highly cross-linked network, replacing the silicon (black dots) as network formers (green dots), or act as glass modifiers (blue dots) that loosely bond with the oxygen (red dots) and disrupt the glass-forming bonds, creating “non-bridging” oxygens. (Adapted from Clinical Applications of Biomaterials 10.1007/978-3-319-56059-5_2)
1 Formers and modifiers When an additive is incorporated into a glass mixture, the ions either become part of the highly cross-linked network, replacing the silicon (black dots) as network formers (green dots), or act as glass modifiers (blue dots) that loosely bond with the oxygen (red dots) and disrupt the glass-forming bonds, creating “non-bridging” oxygens. (Adapted from Clinical Applications of Biomaterials 10.1007/978-3-319-56059-5_2)“There’s a certain sweet spot where you get the right amount [of waste additives] to form a very durable glass,” explains Carolyn Pearce from the Pacific Northwest National Laboratory in the US, who is studying the kinetics of radionuclide stability in waste forms. “If you add in too much, you start pushing the system to form crystalline phases, which is problematic, because then you have multi-phase glass, which is not as durable as a homogeneous single-phase glass.”
Pearce says the waste at Hanford contains “virtually every element in the periodic table in some form or another” and is stored as a liquid, sludge or salt cakes, which makes it more difficult to predict the most stable glass composition. “There’s a lot of modelling that goes into designing the glass-forming elements that will be added. They’ll characterize what’s in the staging tank waiting to go in the facility, and then design the composition of the glass based on that chemistry.”
The use of vitrification for nuclear waste is supported by the stability of natural glasses that have been around for millennia, such as igneous glass, fulgurites (also known as “fossilized lightning”) and glass in meteorites. “In theory, radioactive elements should be released at the same rate as the glass itself dissolves, and we know that glass is highly durable, because we can see volcanic glasses that were made millions of years ago still sitting around today,” says Thorpe. But it isn’t easy to prove that vitrified waste will survive the 60,000 to millions of years necessary for radioactive waste to fully decay – iodine-129, for example, has a half-life of more than 15 million years.
When glass is in contact with water or water vapour, it begins to very slowly deteriorate. First, the alkali metals (sodium or potassium) leach out. The glass networks then start to break down, releasing silicates (and also borates in the case of borosilicate glass) that subsequently form an amorphous gel layer on the glass surface. This becomes dense over time, creating an outer “passivation” layer that can also contain secondary crystallized phases – compounds that form from the surface recrystallization of material that has been released from the bulk glass. At this point, further corrosion is limited by the ability of elements to migrate through this coating.
But if conditions change, or certain mineral species are present, the passivation layer can break down too. “Studies have highlighted elements of concern that could be involved in something called rate resumption, which is where some of the secondary mineral precipitates – particularly iron and magnesium zeolites – have been implicated in the rate of glass dissolution speeding up,” explains Thorpe (figure 2).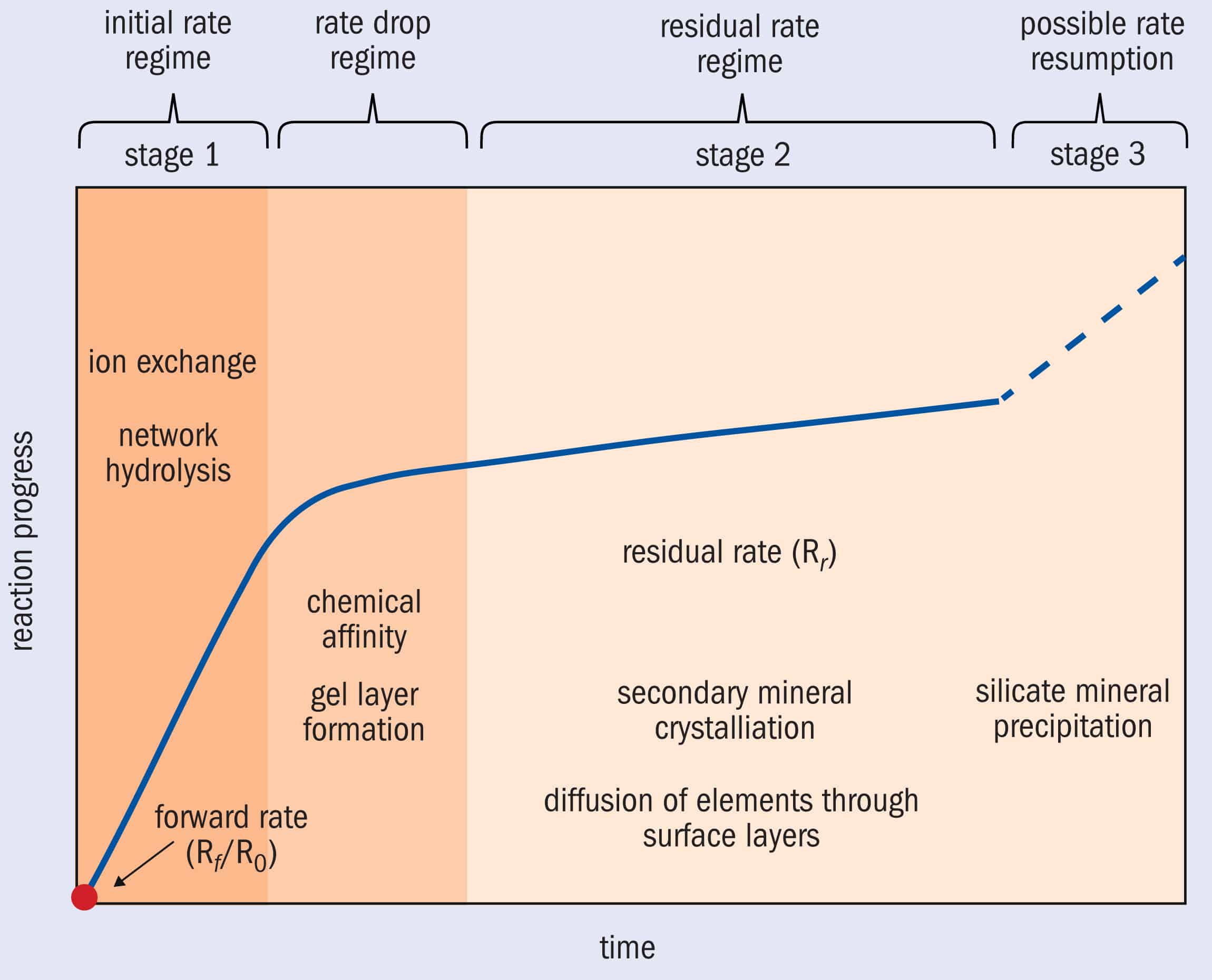 2 Stages of corrosion Glass corrosion occurs in three stages. Stage 1 – The “initial rate regime” involves H3O+ ions diffusing into the glass, displacing network-modifying alkali ions. At the same time, hydrolysis of the glass network releases silicon and other network-forming ions if present. The “rate drop regime” begins once sufficient silica has been freed to form an amorphous gel layer on the glass surface. Stage 2 – The gel “passivation” layer – which densifies over time and can crystallize into secondary mineral phases – slows the rate of dissolution as the ions have to diffuse through it to corrode the glass. This “residual rate regime” can continue indefinitely. Stage 3 – Finally, “rate resumption” occurs if conditions change or certain mineral species are present, such as iron, and the passivation layer breaks down. (Adapted from original by Clare Thorpe)
2 Stages of corrosion Glass corrosion occurs in three stages. Stage 1 – The “initial rate regime” involves H3O+ ions diffusing into the glass, displacing network-modifying alkali ions. At the same time, hydrolysis of the glass network releases silicon and other network-forming ions if present. The “rate drop regime” begins once sufficient silica has been freed to form an amorphous gel layer on the glass surface. Stage 2 – The gel “passivation” layer – which densifies over time and can crystallize into secondary mineral phases – slows the rate of dissolution as the ions have to diffuse through it to corrode the glass. This “residual rate regime” can continue indefinitely. Stage 3 – Finally, “rate resumption” occurs if conditions change or certain mineral species are present, such as iron, and the passivation layer breaks down. (Adapted from original by Clare Thorpe)
 2 Stages of corrosion Glass corrosion occurs in three stages. Stage 1 – The “initial rate regime” involves H3O+ ions diffusing into the glass, displacing network-modifying alkali ions. At the same time, hydrolysis of the glass network releases silicon and other network-forming ions if present. The “rate drop regime” begins once sufficient silica has been freed to form an amorphous gel layer on the glass surface. Stage 2 – The gel “passivation” layer – which densifies over time and can crystallize into secondary mineral phases – slows the rate of dissolution as the ions have to diffuse through it to corrode the glass. This “residual rate regime” can continue indefinitely. Stage 3 – Finally, “rate resumption” occurs if conditions change or certain mineral species are present, such as iron, and the passivation layer breaks down. (Adapted from original by Clare Thorpe)
2 Stages of corrosion Glass corrosion occurs in three stages. Stage 1 – The “initial rate regime” involves H3O+ ions diffusing into the glass, displacing network-modifying alkali ions. At the same time, hydrolysis of the glass network releases silicon and other network-forming ions if present. The “rate drop regime” begins once sufficient silica has been freed to form an amorphous gel layer on the glass surface. Stage 2 – The gel “passivation” layer – which densifies over time and can crystallize into secondary mineral phases – slows the rate of dissolution as the ions have to diffuse through it to corrode the glass. This “residual rate regime” can continue indefinitely. Stage 3 – Finally, “rate resumption” occurs if conditions change or certain mineral species are present, such as iron, and the passivation layer breaks down. (Adapted from original by Clare Thorpe)One of the methods Thorpe and Pearce use to understand these mechanisms is accelerated testing of newly formed glass. “In the laboratory, to speed up the reaction we [flatten] the glass to increase the surface area, and we increase the temperature, typically up to 90 °C,” says Thorpe. “This is really effective for ranking glasses – saying this one’s more durable than this one – but not great for determining the actual dissolution rate in a complex natural environment.”
Instead, researchers have turned to analogue glasses already in existence. “Borosilicate glasses have only been around for about 100 years. We have some data on how they behave long term, but nothing stretching out to the kinds of timescales that we need for thinking about radioactive waste storage,” says Thorpe. Natural glasses are not always a suitable comparison as they tend to be low in alkali elements, which are commonly found in nuclear-waste glasses and will impact their properties – so the other option has been archaeological glasses. While their compositions are not identical to waste glass, they do contain a variety of elements. “Just having these different chemistries really allows us to look at the role that this plays in terms of alteration,” says Pearce.
Glass from the past
Before discovering how to create glass, humans used natural glass for both its strength and beauty. One example is the pectoral, or brooch, found in the tomb of Tutankhamun. Placed on the chest of the mummy, it contains a piece of pale-yellow natural glass shaped into a scarab beetle at least 3300 years ago. The glass came from the Libyan desert, with recent research attributing its formation to a meteorite impact 29 million years ago. Scientists reached this conclusion because of the presence of zirconium silicate crystals within the glass, which come from the mineral reidite that is formed at high pressure (Geology 47 609).
“The earliest production of glass on a regular basis is around 1600 BCE,” says Andrew Shortland, an archaeological scientist at Cranfield University in the UK. “The most spectacular glass object of all, without doubt, is the death mask of Tutankhamun in the Cairo [Museum] catalogue.”
Over the last century archaeologists have disagreed over where glass was first manufactured on a large scale, with northern Syria and Egypt both being prime candidates. “I’d say that at the moment it is too close to call,” says Shortland. The glasses excavated are soda-lime silicate glasses – not too different to the glass we still use in our windows. These were produced using silicate minerals with a “flux” containing soda (Na2CO3), which lowers the melting point to an attainable smelting temperature, and lime (CaCO3) to make the glass harder and chemically more durable. “The silica in these early glasses comes from crushed quartz, which was used because it’s very clean, very low in iron, titanium and other things that colour the glass.”
The problem of glass corrosion is familiar to archaeological conservators who aim to stabilize glass when freshly excavated or stored in museums. “Moisture, obviously, is the worst thing for glass,” says Duygu Çamurcuoğlu, senior objects conservator at the British Museum in London. “If not looked after well, moisture will start attacking and dissolving the glass.” Çamurcuoğlu explains that the beautiful iridescent surface archaeological glasses display is often made up of nearly 90% silicate because other ions, particularly the alkali ions, will have been removed by corrosion.
Archaeological analogues
The key to using archaeological glasses as an analogue for vitrified nuclear waste is having a good knowledge of the environmental conditions the objects have experienced. Trouble is, that gets harder the older the glass is. “Something that’s 200 years old might actually be more useful,” explains Thorpe, “because we can pin down exactly the full climate records.” By comparing archaeological samples to vitrified waste, Thorpe and colleagues are able to validate some of the mechanisms they are seeing in their accelerated high-temperature testing, thereby confirming whether or not they have similar processes and minerals forming, and that there’s nothing they’ve overlooked. (Courtesy: Dr Clare Thorpe)
(Courtesy: Dr Clare Thorpe)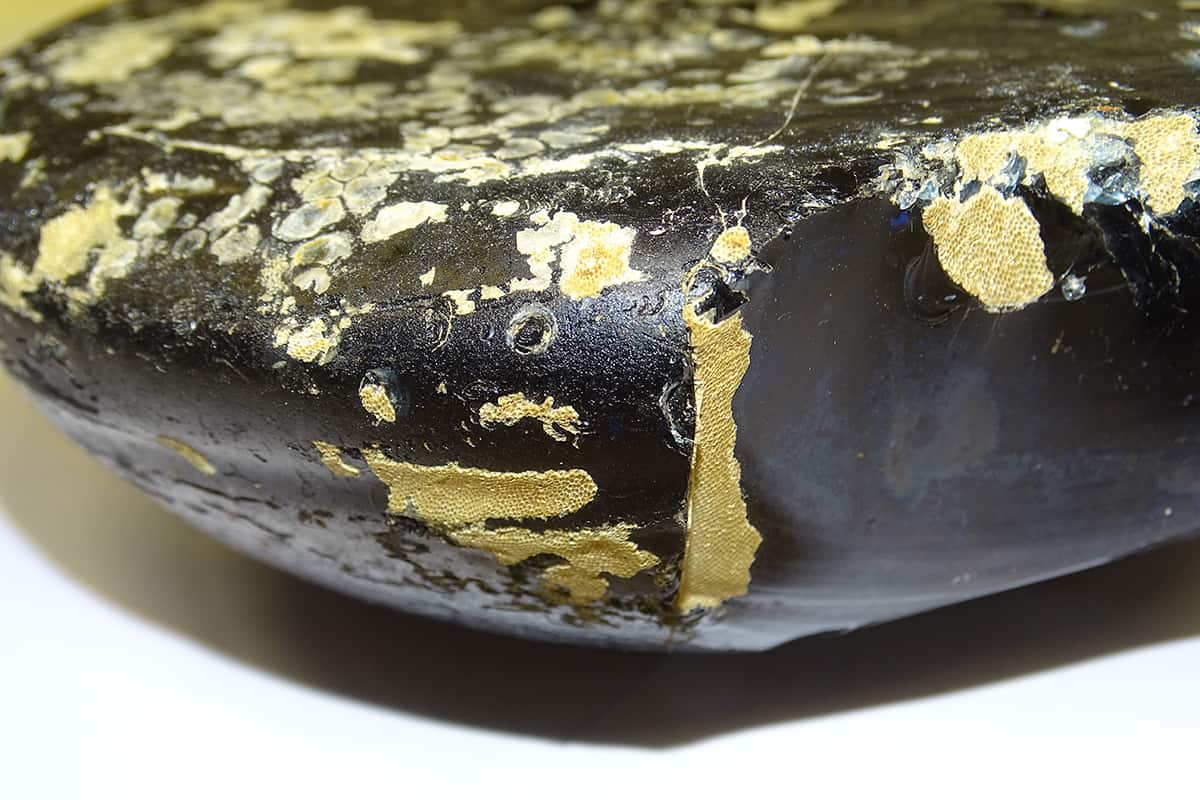 Calculating corrosion These and many more 256-year-old glass ingots were found in a shipwreck off the coast of Margate, UK. We have 200 years of records of the local water temperatures and salinity, which makes it easier to use as a comparison to nuclear waste glasses. (Courtesy: Dr Clare Thorpe)
Calculating corrosion These and many more 256-year-old glass ingots were found in a shipwreck off the coast of Margate, UK. We have 200 years of records of the local water temperatures and salinity, which makes it easier to use as a comparison to nuclear waste glasses. (Courtesy: Dr Clare Thorpe)
 (Courtesy: Dr Clare Thorpe)
(Courtesy: Dr Clare Thorpe) Calculating corrosion These and many more 256-year-old glass ingots were found in a shipwreck off the coast of Margate, UK. We have 200 years of records of the local water temperatures and salinity, which makes it easier to use as a comparison to nuclear waste glasses. (Courtesy: Dr Clare Thorpe)
Calculating corrosion These and many more 256-year-old glass ingots were found in a shipwreck off the coast of Margate, UK. We have 200 years of records of the local water temperatures and salinity, which makes it easier to use as a comparison to nuclear waste glasses. (Courtesy: Dr Clare Thorpe)In Shortland’s experience, the precise local environmental conditions can make a big difference to the length of time glass survives. He remembers using scanning-electron microscopy to analyse glass from the Late Bronze Age city of Nuzi, near Kirkuk in Iraq, originally excavated in the 1930s. “We noticed that some of the glass was perfectly preserved, had beautiful colour, and was robust, while other pieces were weathered and gone completely.” But, he explains, the samples were often found in the same houses in nearby rooms. “We were dealing with micro-environments.” A minor difference in the amount of moisture over 3000 years created very different weathering patterns, as they found (Archaeometry 60 764).
Of course, the sort of glass artefacts found in Nuzi or elsewhere are much too precious to be given to nuclear-waste scientists for testing, but there are many less-rare pieces of archaeological glass available. Thorpe is looking at several well-characterized archaeological sites where material may provide useful analogues, such as slag – the silicate-glass waste product formed during iron smelting. Slag blocks had been incorporated into a wall at the Black Bridge foundry, a site within the town of Hayle in Cornwall, UK, constructed around 1811 (Chem. Geol. 413 28). “They’re fairly analogous to some of the plutonium-contaminated material when they are vitrified,” she explains. “You can be sure that they’ve been exposed to either the air or the estuary that they’ve sat in for 250 years.” She has also investigated 265-year-old glass ingots from the Albion shipwreck off the coast of Margate, UK, where there are comprehensive records of water temperatures and salinity dating back 200 years.
Thorpe and others have also been considering the impact of metals on glass stability. “We’re very interested in the role of iron as it’s going to be present because of the canisters [holding the vitrified waste]. In the natural analogue sites, it’s present because a lot of the time the glass is in soil or, in the case of the slags, surrounded by iron-rich material.” The worry is that positive iron ions, leaching from the glass or surroundings, scavenge negatively charged silicates from the glass’s surface gel layer. This would precipitate out iron silicate minerals, potentially disrupting the passification layer and triggering rate resumption. This effect has been seen in a number of laboratory studies (Environ. Sci. Technol. 47 750) but Thorpe wants to see it happening in the field at low temperatures because the thermodynamics are very different to accelerated testing. So far, they don’t have evidence that this is occurring with vitrified nuclear waste and are confident that with or without the presence of iron, these glasses are highly durable. But it is still important to understand the processes that might affect the rate at which corrosion happens.
A biological challenge
An analogue glass that Pearce and colleagues have been studying comes from the Broborg pre-Viking hillfort in Sweden, which was occupied around 1500 years ago. It contains vitrified walls that Pearce thinks were purposefully constructed, rather than being the results of accidental or violent destruction of the site. The granite walls were strengthened by melting amphibolite rocks that contain largely silicate minerals, to form a vitrified mortar surrounding the granite boulders. “We know exactly what’s happened to the glass in terms of what temperatures it’s been exposed to, and the amount of rainfall, through records in Sweden going back those 1500 years,” says Pearce. Glass walls An archaeological dig at the Broborg pre-Viking hillfort in Sweden has revealed walls fortified by melting silicate-containing rock to form a glass mortar around the granite. (Courtesy: Mia Englund, The Archaeologists)
Glass walls An archaeological dig at the Broborg pre-Viking hillfort in Sweden has revealed walls fortified by melting silicate-containing rock to form a glass mortar around the granite. (Courtesy: Mia Englund, The Archaeologists)
 Glass walls An archaeological dig at the Broborg pre-Viking hillfort in Sweden has revealed walls fortified by melting silicate-containing rock to form a glass mortar around the granite. (Courtesy: Mia Englund, The Archaeologists)
Glass walls An archaeological dig at the Broborg pre-Viking hillfort in Sweden has revealed walls fortified by melting silicate-containing rock to form a glass mortar around the granite. (Courtesy: Mia Englund, The Archaeologists)Using electron microscopy to study the Broborg glass, the researchers were surprised to find the surface exposed to the environment covered in bacteria, fungi and lichens. Pearce’s team is now trying to understand the implications of such biological activity on the glass’s stability. The site contains several different glass compositions and they found that samples with more iron showed more evidence of microbial colonization (possibly due to the larger number of organisms able to metabolize iron) and more evidence of physical damage such as pitting.
While it seems as though certain organisms can thrive in these harsh conditions, and may even extract elements from the material, Pearce explains that it’s also possible that a biofilm provides a protective layer. “The bacteria like to live in relatively unchanging conditions, as all living organisms are engaged in homeostasis, and so they try to regulate the pH and the water content around them.” Her team is now trying to determine what role the biofilm plays and how that relates to the glass composition (npj Materials Degradation 5 61).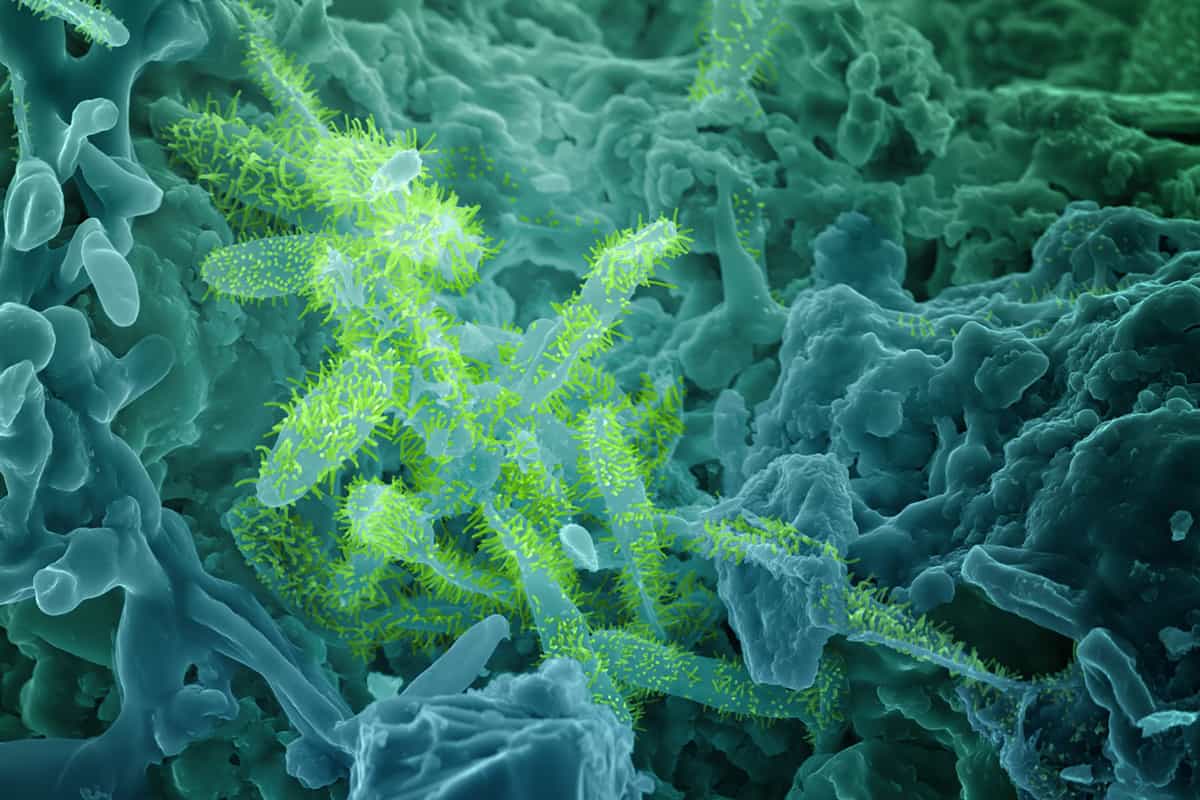 Living layer Scanning electron microscopy of the naturally created glass used to fortify walls at the Broborg pre-Viking hillfort in Sweden reveals that the exposed surface of this glass is covered in micro-organisms, with more microbes where the iron content is higher. (Courtesy: Bruce Arey, PNNL)
Living layer Scanning electron microscopy of the naturally created glass used to fortify walls at the Broborg pre-Viking hillfort in Sweden reveals that the exposed surface of this glass is covered in micro-organisms, with more microbes where the iron content is higher. (Courtesy: Bruce Arey, PNNL)
 Living layer Scanning electron microscopy of the naturally created glass used to fortify walls at the Broborg pre-Viking hillfort in Sweden reveals that the exposed surface of this glass is covered in micro-organisms, with more microbes where the iron content is higher. (Courtesy: Bruce Arey, PNNL)
Living layer Scanning electron microscopy of the naturally created glass used to fortify walls at the Broborg pre-Viking hillfort in Sweden reveals that the exposed surface of this glass is covered in micro-organisms, with more microbes where the iron content is higher. (Courtesy: Bruce Arey, PNNL)The key problem faced by those looking to create the most stable nuclear-waste glasses is that of longevity. But for archaeological conservators trying to stabilize deteriorating glass, they have a more urgent challenge, which is to remove moisture and therefore stop the glass from cracking and shattering. Archaeological glass can be consolidated with acrylic resin, applied on top of the iridescent corrosion layer. “It’s actually [part of] the glass itself, so it should be protected,” says Çamurcuoğlu.
Despite how long we’ve been using glass, there is still a long way to go in fully understanding how its structure and composition impacts its stability. “It amazes me that we still can’t guess the melting temperature of a glass from its composition entirely accurately. Very small amounts of additional elements can have huge effects – it really is a bit of a dark art,” muses Thorpe.
Her work at Sheffield will continue, with some projects handed down to her that have been running for over 50 years. The Ballidon Quarry in Derbyshire, UK, for example, hosts one of the longest running “glass burial” experiments in the world. The aim is to test the degradation of archaeological glasses under the sort of alkaline conditions that vitrified nuclear waste will experience, alongside waste encased in cement (J. Glass Stud. 14 149). The experiment is intended to run for 500 years. Whether the university itself will last that length of time remains to be seen, but as for the nuclear waste they are working to protect us from, it certainly will endure.
Rachel Brazil is a science writer based in London, UK, Twitter @rachelbbrazil
from physicsworld.com 29/6/2022

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου