Quantum effects help make DNA unstable
14 Jun 2022 Isabelle Dumé

Quantum effects play a hitherto unexpected role in creating instabilities in DNA – the so-called “molecule of life” that provides instructions for cellular processes in all living organisms. This conclusion, based on work by researchers at the University of Surrey in the UK, goes against long-held beliefs that quantum behaviour is not relevant in the wet, warm environment of cells, and could have far-reaching consequences for models of genetic mutation.
The two strands of the DNA double helix are linked together by hydrogen bonds between the DNA bases. There are typically four different bases, called Guanine (G), Cytosine (C), Adenine (A) and Thymine (T). In the standard configuration, A always bonds to T while C always bonds to G. However, if the protons (nuclei of the hydrogen atoms) that make up the bonds hop from one strand of DNA to the other then a genetic mutation can occur.
This effect was predicted back in 1952, when James Watson and Francis Crick drew on work by Rosalind Franklin and Maurice Wilkins to uncover DNA’s helical structure. However, it is only now that this DNA bond modification process has been accurately quantified, and its quantum element understood.
Proton transfer along DNA hydrogen bonds
In their work, Louie Slocombe, Marco Sacchi, Jim Al-Khalili and colleagues used sophisticated computer models to show that DNA bond modification stems from the protons’ ability to transfer along the hydrogen bonds that form between the G-C bases (more easily than in A-T). As the protons hop from one side of the DNA strand to the other, a mismatch occurs if one of these hops happens just before the DNA strand cleaves, or “unzips”, as part of the process it undergoes to copy itself.
To pin down what makes protons hop along DNA strands, the researchers first used a computational chemistry approach called density functional theory to model the hydrogen bonds, then calculated the proton dynamics as an open quantum system influenced by its surrounding cellular environment. They discovered that protons can easily quantum tunnel through a potential barrier from one DNA strand to the other and that this tunnelling rate is so fast that thermal equilibrium is quickly reached, giving a constant tautomer population over biological timescales.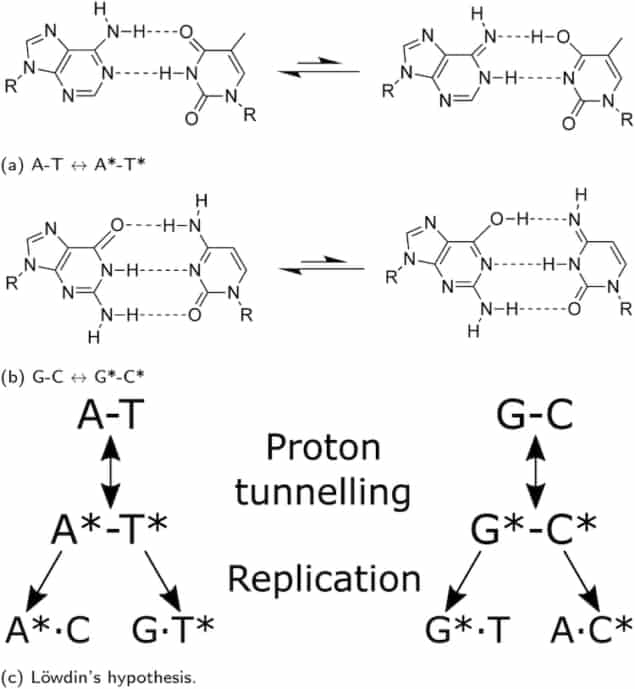 Schematic representation of Löwdin’s hypothesis of spontaneous mutagenesis. A double proton tunnelling process converts canonical into tautomeric base pairs. (Courtesy: L Slocombe)
Schematic representation of Löwdin’s hypothesis of spontaneous mutagenesis. A double proton tunnelling process converts canonical into tautomeric base pairs. (Courtesy: L Slocombe)
 Schematic representation of Löwdin’s hypothesis of spontaneous mutagenesis. A double proton tunnelling process converts canonical into tautomeric base pairs. (Courtesy: L Slocombe)
Schematic representation of Löwdin’s hypothesis of spontaneous mutagenesis. A double proton tunnelling process converts canonical into tautomeric base pairs. (Courtesy: L Slocombe)Quantum effects do matter
Until now, it was thought that any such quantum behaviour should wash out quickly in the noisy conditions that prevail inside cells, and thus would not play any physiological role. However, Slocombe explains that the DNA system is so sensitive to the hydrogen bond arrangement that quantum effects do matter. Indeed, even the tiny rearrangement of a pair of protons can affect how DNA replicates on the macroscopic scale.
“The topic is exciting to study since it involves the combination of techniques and ideas from different realms of science,” Slocombe tells Physics World. “Typically, these are not congruent and we require them to be so to model the system accurately. We require knowledge of both chemistry and physics to model the systems and in addition we need to know about biology, how DNA replicates and the implications for when it mismatches.”READ MORE

The researchers, who report their work in Communication Physics, express hope that their study “is the first of many” on this topic. “What most interests us,” Slocombe adds, “is what happens at the exact moment of the DNA cleaving and how the timescale of this interaction interplays with the fast timescale of the hydrogen transfer.”
Other questions include whether using ATGC bases rather than alternative forms of DNA confers some evolutionary benefit, since the former are relatively unstable. Another is whether this instability leads to mutation, thus driving the process of evolution. “It would be interesting to understand if there are any DNA repair pathways specifically designed to catch these types of errors,” Slocombe concludes.

Isabelle Dumé is a contributing editor to Physics World
from physicsworld.com 18/6/2022
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου