Researchers use 3D printing to grow full-thickness skin in the lab
30 Nov 2021
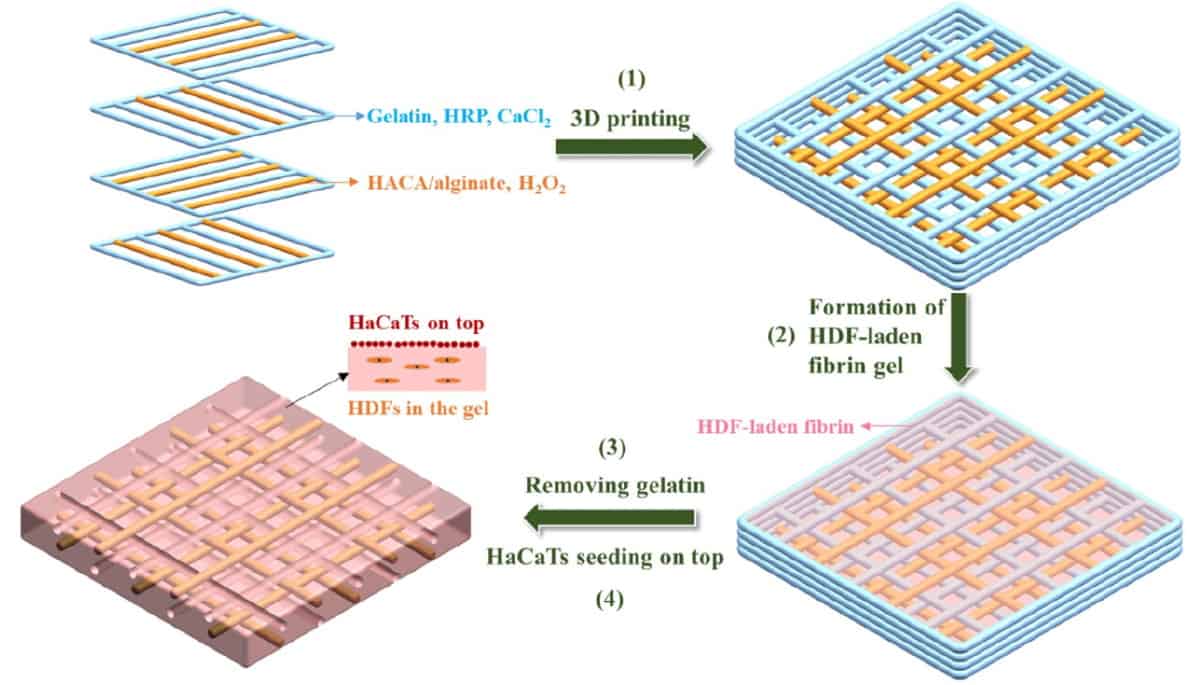
Fabrication of a double-layered skin model using human dermal fibroblasts (HDFs) and an immortal human keratinocyte cell line (HaCaTs). (Courtesy: Biofabrication 10.1088/1758-5090/ac2ef8)
Skin is the body’s first line of defence against toxins, radiation and harmful substances. It has at least six functions, regenerates itself approximately once each month, and consists of up to seven layers of tissue. It’s no wonder, then, that researchers and clinicians are interested in producing this remarkable organ in the lab so that it can be used to repair injuries caused by burns, surgery or disease.
While scientists can grow the epidermis, the outer layer of skin, in the lab, a major challenge for researchers today is growing functional, full thickness skin, which provides strength and flexibility and contains blood vessels. Researchers at the Intelligent Polymer Research Institute at the University of Wollongong in Australia are using 3D printing techniques to tackle this problem.
“There have been tremendous advances in biomaterials science and cell biology with respect to tissue engineering over the past two decades,” says Gordon Wallace, director of the Intelligent Polymer Research Institute. “Advances in 3D biofabrication enable us to converge knowledge in these two areas and to arrange existing materials in a way that dramatically enhances performance.”
Wallace is a senior author on a recent study applying 3D printing techniques to generate a skin-like structure that supports the growth of dermal fibroblasts found in the inner layers of skin. The study, published in the journal Biofabrication, presents a 3D printing platform that could be explored to engineer functional skin tissue.
Lab-grown skin
The researchers’ interest in skin regeneration is driven by collaborators at St. Vincent’s Hospital in Melbourne and at the Royal Perth Hospital and Western Australia Burns Service. Wallace attributes most of the work in the study to Ying Zhou, a highly talented PhD student who led the project.
“To facilitate regeneration of skin requires a 3D structure containing materials that can support development of appropriate cells through the provision of a composition and physical environment that promotes healing,” Wallace explains. “It was Ying’s insights and technical ability that brought this all to fruition.”
The team used a custom-designed ink for 3D printing the skin-like structures. Developed by the group last year, the ink is composed of catechol-hyaluronic acid (HACA), a polymer used in biological, stem cell and tissue engineering settings, and alginate, a compound found in the cell walls of brown algae that’s used in a variety of pharmaceuticals.
The HACA–alginate ink provides structural support with specific attributes while providing a balance between mechanical properties and cytocompatibility (it is not harmful to cells). The resulting printed hydrogel skin scaffold, verified using nuclear magnetic resonance and ultraviolent–visible spectroscopy, recovers after bending and has high elasticity and toughness.
“The importance of the elasticity is in providing an appropriate physical environment to facilitate cell proliferation, migration, reorganization and differentiation during the regeneration process,” says Zhilian Yue, another senior author on the Biofabrication study.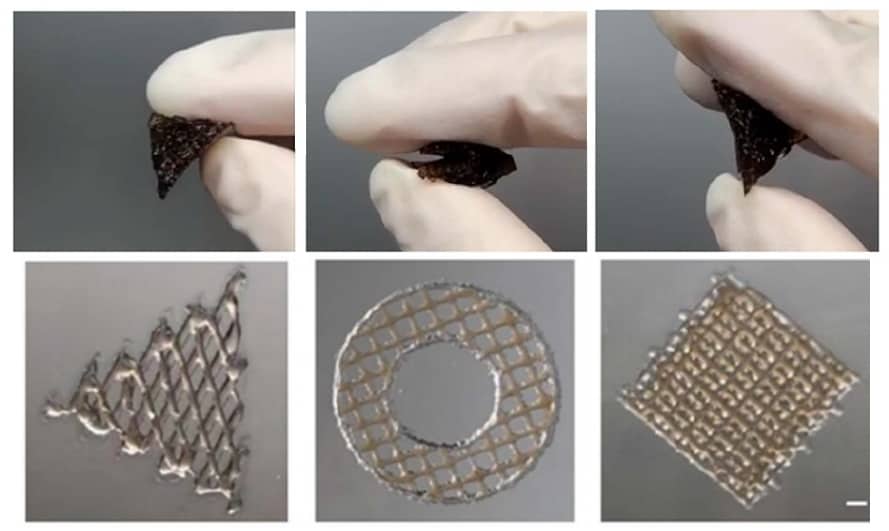 Flexible designs: The printed scaffolds were highly elastic and could recover after bending (top row); printed structures of various patterns (bottom row). (Courtesy: Biofabrication 10.1088/1758-5090/ac2ef8)
Flexible designs: The printed scaffolds were highly elastic and could recover after bending (top row); printed structures of various patterns (bottom row). (Courtesy: Biofabrication 10.1088/1758-5090/ac2ef8)
 Flexible designs: The printed scaffolds were highly elastic and could recover after bending (top row); printed structures of various patterns (bottom row). (Courtesy: Biofabrication 10.1088/1758-5090/ac2ef8)
Flexible designs: The printed scaffolds were highly elastic and could recover after bending (top row); printed structures of various patterns (bottom row). (Courtesy: Biofabrication 10.1088/1758-5090/ac2ef8)The multi-material scaffold, rather mimicking the elasticity of skin, encourages the development of tissue that can integrate with existing skin – the scaffold includes microchannels made from gelatin that can be used to mimic blood vessels and other vasculature and further facilitate cell growth – and that can be integrated with a cell-friendly matrix that promotes the growth of human dermal fibroblasts.
Introducing cells to scaffolds
With the skin-like scaffolds built, the researchers introduced the scaffolds to skin cells encapsulated in fibrin gel. They validated the self-assembly of fibrinogen, a soluble protein that’s converted to fibrin at wound sites in the presence of clotting enzymes, using scanning electron microscopy. The team’s initial analyses of the lab-grown skin also included measuring the thickness of the epidermal layer and analysing the differentiation of the epidermal layer and extracellular matrix deposition of the dermal layer using histology analyses and immunostaining.READ MORE

“This work indicates that multicellular systems using skin as a model can be developed within a 3D structure comprised of commonly available biomaterial. These biomaterials work together to produce a tough and elastic hydrogel framework with built-in micro channels, to support cells in their specific environment as dictated by the targeted application,” Yue says.
Now, the researchers are working with their collaborators to determine the best way to deliver and use this multi-material skin regeneration platform in vivo, including tailoring its structure and composition to different types of injury.
Catherine Steffel is a science writer based in Madison, Wisconsin. Catherine was previously a PhD student contributor to Physics World.
from physicsworld.com 29/6/2022
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου