Machine learning framework classifies pneumonia on chest X-rays
24 Apr 2023 Tami Freeman
Test data Chest X-ray images showing examples of normal lung (left), bacterial pneumonia (centre), and viral pneumonia (right). (Courtesy: Mach. Learn.: Sci. Technol. 10.1088/2632-2153/acc30f)
Pneumonia is a potentially fatal lung infection that progresses rapidly. Patients with pneumonia symptoms – such as a dry, hacking cough, breathing difficulties and high fever – generally receive a stethoscope examination of the lungs, followed by a chest X-ray to confirm diagnosis. Distinguishing between bacterial and viral pneumonia, however, remains a challenge, as both have similar clinical presentation.
Mathematical modelling and artificial intelligence could help improve the accuracy of disease diagnosis from radiographic images. Deep learning has become increasingly popular for medical image classification, and several studies have explored the use of convolutional neural network (CNN) models to automatically identify pneumonia from chest X-ray images. It’s critical, however, to create efficient models that can analyse large numbers of medical images without false negatives.
Now, K M Abubeker and S Baskar at the Karpagam Academy of Higher Education in India have created a novel machine learning framework for pneumonia classification of chest X-ray images on a graphics processing unit (GPU). They describe their strategy in Machine Learning: Science and Technology.
Training data optimization
The performance of a deep learning classifier relies on both the neural network model and the quality of the data used to train the network. For medical imaging, the lack of a large enough dataset is a primary cause of subpar performance. To address this shortfall, the researchers used data augmentation, in which new training data are synthesized from existing data (for example via image rotations, shifts and crops) to make the dataset more comprehensive and diverse.
Another method employed to address the lack of appropriate training data is transfer learning – improving a model’s capacity to learn a new task using existing knowledge obtained while performing a related task. In the first phase of their study, Abubeker and Baskar used transfer learning to train nine state-of-the-art neural CNN models to assess whether or not a chest X-ray portrays pneumonia.
For the experiments, they used chest X-ray images from public RSNA Kaggle datasets, including images for training (1341 categorized as normal, 1678 as bacterial pneumonia and 2197 as viral pneumonia), testing (234 normal, 184 bacterial pneumonia, 206 viral pneumonia) and validation (76 normal, 48 bacterial pneumonia, 56 viral pneumonia). Applying geometric augmentation to the dataset expanded it to a total of 2571 normal, 2019 bacterial and 2625 viral pneumonia images.
Based on performance measures including accuracy, recall and the area under the ROC curve (AUROC, a metric summarising performance over several thresholds), the researchers chose the three top performing CNN models – DenseNet-160, ResNet-121, and VGGNet-16 – for retraining using an ensemble technique.
Ensemble strategy
Rather than relying on a single machine learning model, ensemble models pool the conclusions of several models to boost performance metrics and minimize errors. The researchers developed a transfer learning-based ensemble strategy called B2-Net and used this with the three selected CNNs to create a final model. They implemented the final B2-Net model on an NVIDIA Jetson Nano GPU computer.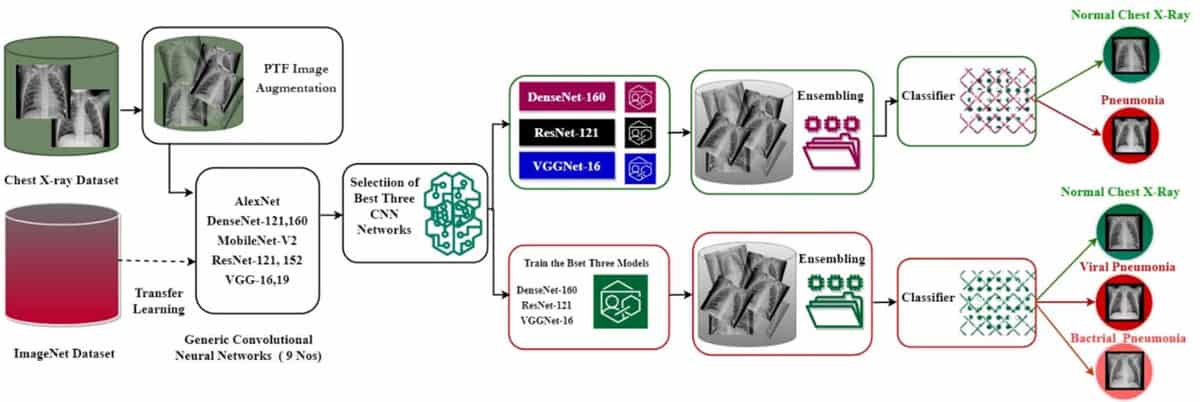 Ensemble approach Graphical representation of the B2-Net model developed for the classification of pneumonia in chest X-ray images. (Courtesy: Mach. Learn.: Sci. Technol. 10.1088/2632-2153/acc30f)
Ensemble approach Graphical representation of the B2-Net model developed for the classification of pneumonia in chest X-ray images. (Courtesy: Mach. Learn.: Sci. Technol. 10.1088/2632-2153/acc30f)
 Ensemble approach Graphical representation of the B2-Net model developed for the classification of pneumonia in chest X-ray images. (Courtesy: Mach. Learn.: Sci. Technol. 10.1088/2632-2153/acc30f)
Ensemble approach Graphical representation of the B2-Net model developed for the classification of pneumonia in chest X-ray images. (Courtesy: Mach. Learn.: Sci. Technol. 10.1088/2632-2153/acc30f)They note that during training, some models performed better in identifying normal X-ray images, while others performed better in identifying viral and bacterial pneumonia samples. The ensemble strategy uses a weighted voting technique to provide each classifier with a specific degree of power based on predefined criteria.
The retrained models demonstrated significant improvements in diagnostic accuracy over the baseline models. Testing the models on a balanced dataset revealed that DenseNet-160, ResNet-121 and VGGNet-16 achieved AUROC values of 0.9801, 0.9822 and 0.9955, respectively. The proposed B2-Net ensemble approach, however, outperformed all three, with an AUROC of 0.9977.
The researchers evaluated and validated B2-Net and the other three models using a subset of around 600 chest X-ray images from the pooled dataset. DenseNet-160 misidentified three of the pneumonia test images, while VGGNet-16 and ResNet-121 misdiagnosed one X-ray image each. Overall, the proposed B2-Net approach outperformed all other models, distinguishing between normal cases, bacterial pneumonia and viral pneumonia in chest X-ray images with 97.69% accuracy and a recall rate (the proportion of true positives among the total number of positives) of 100%.READ MORE

Abubeker and Baskar explain that while the false negative rate is the most critical criterion for a medical image classifier, the proposed B2-Net model provides the best alternative for real-time clinical applications. “This approach, particularly during the present worldwide Covid-19 outbreaks, could assist radiologists in swiftly and reliably diagnosing pneumonia, allowing for early treatment,” they write.
Next, they plan to expand their model to classify more lung disorders, including TB and Covid-19 variants.

Tami Freeman is an online editor for Physics World
FROM PHYSICSWORLD.COM 27/4/2023

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου