Ionocaloric cooling makes a new type of refrigerator
07 Feb 2023 Isabelle Dumé
Ionocaloric cooling could help phase out refrigerants that contribute to global warming. (Courtesy: Jenny Nuss/Berkeley Lab)
A new refrigeration method dubbed “ionocaloric cooling” could one day replace traditional systems based on vapour compression, reducing the need for gases that harm the Earth’s atmosphere and contribute to climate change. The method, developed by researchers at the Lawrence Berkeley National Laboratory (LBNL) in the US, takes advantage of the ways that energy is stored or released when a material changes phase, such as from a solid to a liquid or vice versa.
Conventional refrigerators and air conditioners are designed to use volatile hydrofluorocarbons, which are extremely powerful greenhouse gases with a global warming potential (GWP) 2000 times greater than carbon dioxide. In such systems, the refrigerant is pumped around a closed loop in which it undergoes a phase change from a liquid to a gas and then back to a liquid. The transition to a gas involves an expansion and requires energy, which the refrigerant acquires by cooling the surroundings on its “cold” side. Heat is then released on the “hot” side when the fluid condenses back to a liquid.
This standard cycle can also be applied to other substances that similarly undergo a phase transition involving the absorption and emission of heat. These alternative substances include electrocaloric and magnetocaloric materials, which switch between two solid phases in the presence of applied electric or magnetic fields. The drawback is that the heating and cooling abilities of electrocaloric and magnetocaloric refrigerants are relatively modest, leading to cooling cycles that are inefficient for widespread practical use.
A third possibility is to use the barocaloric effect, which occurs when the material being compressed and expanded is a solid rather than a liquid or gas. For most barocaloric materials, however, this effect is very small at ambient temperatures and pressures.
A completely new caloric effect
The new technique invented by Drew Lilley and Ravi Prasher at LBNL makes use of an entirely different caloric effect. It works by adding salt to a solid, which makes the solid “want” to be a liquid in the same way as adding salt to a cold, icy road transforms the ice into slush.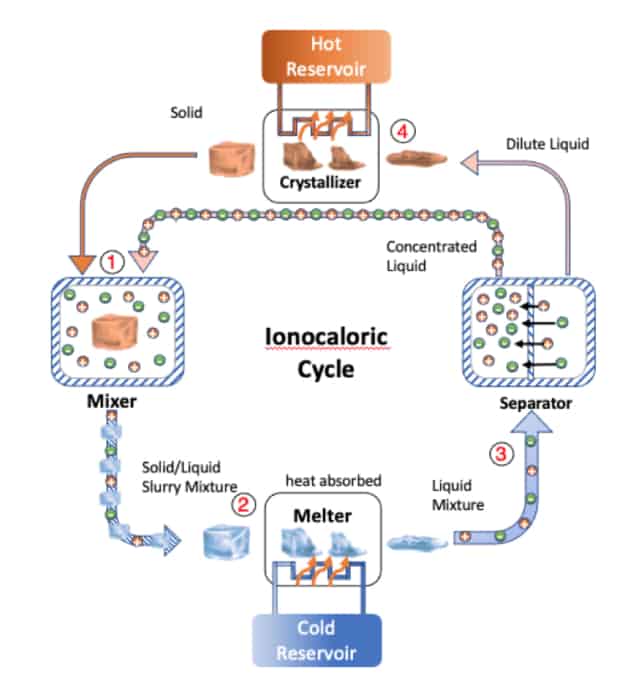 Salty ice: The ionocaloric cycle. (Courtesy: Drew Lilley/Berkeley Lab)
Salty ice: The ionocaloric cycle. (Courtesy: Drew Lilley/Berkeley Lab)
 Salty ice: The ionocaloric cycle. (Courtesy: Drew Lilley/Berkeley Lab)
Salty ice: The ionocaloric cycle. (Courtesy: Drew Lilley/Berkeley Lab)“To become a liquid, the solid must melt, which means it must absorb energy,” Lilley explains. “If you prevent the solid from absorbing energy from its surrounding, it will ‘steal’ energy from itself, so cooling the whole material down (see steps 1 to 2 in the image above). Once it has cooled down, the solid can continue melting, but at a lower temperature, and absorbs energy from its surroundings. This leads to refrigeration (steps 2 to 3 in the diagram).”
Lilley goes on to explain that the mechanism for the phase and temperature change in this “ionocaloric” cycle is the flow of electrically charged atoms or molecules – ions – when a current is applied to the system. If the ions are later removed from the liquid that contains dissolved salt (steps 3 to 4 in the diagram) the reverse effect occurs: the substance no longer “wants” to be a liquid, so it becomes a solid. To do so, it must crystallize and release energy, but if it is prevented from exchanging energy with its surroundings, it will instead release the energy to itself and heat up. Once heated, it will continue releasing energy by crystallizing and give up this heat to its environment.
In a series of experiments designed to test the refrigeration capabilities of this solvent-salt mixing process, Lilley and Prasher found that the temperature decreased by up to 28 °C using less than 1 V of applied current. They also observed variations in entropy (the physical entity used to estimate the effectiveness of a cooling principle) as large as 500 J K-1 kg-1. This is greater than the variations observed in magnetocaloric and electrocaloric materials and similar to that of the best barocaloric material (plastic crystals of neopentylglycol). It also compares well to today’s refrigerants.
A slow, salty cycle
The salt the researchers used is made from iodine and sodium, and they mixed it with ethylene carbonate – a common organic solvent that is, incidentally, a common additive in lithium-ion battery electrolytes. The resulting ethylene carbonate–sodium iodide (EC-NaI) mixture is, they say, CO2-negative, environmentally benign, non-hazardous, zero-GWP, nontoxic and non-flammable.READ MORE

“Our technology is sustainable [and] does not employ extreme fields – we only need to apply around 1 V,” Lilley tells Physics World. He adds that the efficiency of the prototype system in their experiment is “four to five times larger than any previous prototypes utilizing solid-state materials” and exhibits “power densities that rival that of vapour compression”.
The main drawback of ionocaloric refrigeration is its slow speed. According to Lilley and Prasher, who published their work in Science, a single cycle can take between five minutes and several hours. Even so, Emmanuel Defay, a researcher at the Luxembourg Institute of Science and Technology who was not involved in the work, is impressed by the potential of this new member of the caloric material family. “It exhibits large efficiency and could be environmentally benign,” he writes in a related Perspectives article. “This is a serious contender for the future of cooling.”
The LBNL researchers’ next step will be to start a company to commercialize their technology. “Hopefully our approach will have a real-world impact on improving refrigeration and heat pumping through efficiency gains and the decarbonization of refrigerants,” Lilley says.

Isabelle Dumé is a contributing editor to Physics World
FROM PHYSIICSWORLD.COM 4/8/2023

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου