Tiny probe measures deep-brain activity from inside a blood vessel
18 Aug 2023
Neural recording across the vessel wall A micro-endovascular probe (yellow) designed for insertion into straight (versus branched) blood vessels is preloaded into a flexible microcatheter (cyan) and selectively injected into the straight vessel by saline flow. (Courtesy: Anqi Zhang, Stanford University)
Brain–machine interfaces (BMIs) provide direct electrical communication between the brain and external electronic systems. As such, BMIs offer potential to restore impaired functionality in patients with paralysis or neurological disorders, by using brain activity alone to directly control prostheses or computer programs, for example, or to modulate nerve or muscle function.
Improvements in function and performance are needed, however. Today, most conventional BMIs measure neural activity at the brain’s surface, which provides limited spatial resolution. The ability to record single neuron activity from deep-brain regions could dramatically improve future BMI technology.
Measurement of single neurons in deep-brain regions is currently achieved by surgically implanting probes into the brain. This is far from ideal, as surgery can damage brain tissue, as well as cause inflammation and scarring, which rapidly degrade device performance. Clearly, there’s a need for a less invasive and longer-lasting approach.
Researchers at Stanford University and Harvard University have developed such a device: an ultrasmall and ultra-flexible micro-endovascular (MEV) neural probe that can be that can be implanted into sub-100-µm-scale blood vessels in the brains of rodents without requiring open-skull surgery. Instead, the device uses the brain’s vascular system as a probe delivery route, inserting the MEV probes via flexible microcatheters. First author Anqi Zhang of Stanford University. (Courtesy: Anqi Zhang)
First author Anqi Zhang of Stanford University. (Courtesy: Anqi Zhang)
 First author Anqi Zhang of Stanford University. (Courtesy: Anqi Zhang)
First author Anqi Zhang of Stanford University. (Courtesy: Anqi Zhang)After inserting the microcatheter into the targeted vessel, the probes are injected into deeper vasculature by saline flow. The probes advance smoothly within the microcatheter into the vessels and remain extended without buckling. The researchers explain that the device’s mesh-like structure relaxes and unfolds after injection, allowing the electrodes to adhere against the inner vessel walls in a similar manner to the deployment of a vascular stent. Once in place, the probe can record neuronal signals across the blood vessel wall without damaging the brain or vasculature.
Anqi Zhang of Stanford University and Charles Lieber, formerly at Harvard University, explain that “the metabolically active central nervous system requires a dense vascular network, so the average neuron is less than 20 µm from the nearest blood vessel. This vasculature thus offers recording probes access to any brain region without damaging the recorded neural circuits.”
Measuring individual neurons
For their study, reported in Science, the researchers created probes containing sixteen 80 µm-long platinum electrodes, distributed over a length of 1 cm to examine multiple brain regions. They initially tested the ability of the MEV probes to record activity in the brains of anaesthetized rats, reporting well-defined signals across the 16 channels.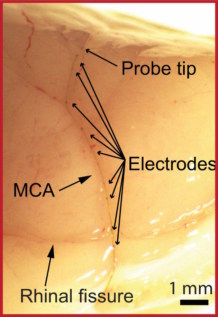 In vivo recording recording Micro-endovascular probe in the middle cerebral artery of a rat brain. (Courtesy: Anqi Zhang, Stanford University)
In vivo recording recording Micro-endovascular probe in the middle cerebral artery of a rat brain. (Courtesy: Anqi Zhang, Stanford University)
 In vivo recording recording Micro-endovascular probe in the middle cerebral artery of a rat brain. (Courtesy: Anqi Zhang, Stanford University)
In vivo recording recording Micro-endovascular probe in the middle cerebral artery of a rat brain. (Courtesy: Anqi Zhang, Stanford University)The team then induced local seizures in the animals by intracortical injection of penicillin to create epilepsy models. The probes accurately recorded bilateral spikes and spike-wave complexes associated with seizure activity following the injection. Simultaneous recording from all 16 channels demonstrated the ability of the MEV probes to locate and track the seizure foci.
To study any short-term effects of the MEV probes, the researchers used laser Doppler flowmetry to monitor cerebral blood flow before and immediately after probe injection. They found that probe implantation did not substantially impact cerebral blood flow. Investigation of chronic effects showed that none of the rats experienced any neurologic deficits, the integrity of the blood–brain barrier was well preserved, there was no increase in vessel wall thickness, and no significant immune response.
“These observations not only demonstrate the minimal invasiveness of the MEV probes, but also indicate major advantages in chronic electrophysiology recording, as the accumulation of glial scar tissue near the brain electrodes is known to cause electrode failure in clinically relevant chronic settings,” the team explains.
The researchers point out that their probes can be selectively implanted into small vessel branches that are not accessible to any existing microcatheters, thus enabling neural recording across vessel walls at single-cell resolution. Zhang tells Physics World that the team plans to improve the probe design and materials to enable better navigation into different vessel branches. In the long term, the researchers hope to use the probes to study the brain and treat brain diseases, and achieve clinical translation for applications in neurology and interventional radiology.READ MORE

Writing in an accompanying commentary in Science, Brian Timko of Tufts University School of Engineering says that this ability to achieve non-invasive, single-neuron recordings is important for studies of deep-brain regions such as the medial temporal lobe, where activity is not spatially clustered and therefore only identifiable at the single-neuron level. He suggests that future studies could answer long-standing questions about how memories are stored and retrieved.
Timko notes that these endothelial probes represent a general platform that in the future could be broadened to incorporate localized stimulation devices. Such stimulation elements might also be used to electroporate the blood vessel wall, enabling localized drug delivery across the blood–brain barrier. He envisions that future development of endovascular probes might ultimately form the foundation for machine interfaces throughout the body.
Cynthia E Keen is a freelance journalist specializing in medicine and healthcare-related innovations.
FROM PHYSICSWORLD.COM 22/8/2023

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου