Light controls a ‘living biomaterial’
18 Sep 2018 Paul Humphreys
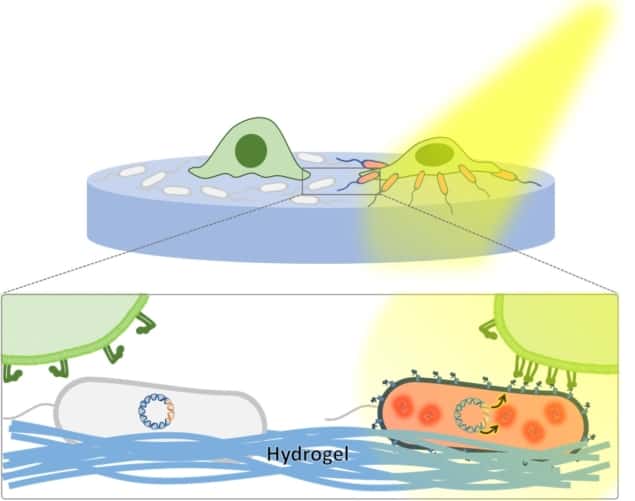 The light-regulated living biomaterial. Light stimulates the display of RGD by E. coli bacteria, enabling interaction with mammalian cells. (Courtesy: Advanced Science 10.1002/advs.201800383/CC BY 4.0)
The light-regulated living biomaterial. Light stimulates the display of RGD by E. coli bacteria, enabling interaction with mammalian cells. (Courtesy: Advanced Science 10.1002/advs.201800383/CC BY 4.0)
Scientists in the Dynamic Biomaterials group at the Leibniz Institute for New Materials have demonstrated a proof-of-concept design for a light-regulated “living biomaterial” (Advanced Science 10.1002/advs.201800383).
An ideal biomaterial for use in medical applications would have the ability to transmit and respond to signals from attached or encapsulated cells, in order to mimic the molecular interactions that occur within living tissues. Recent advances in this area include the timed release of sequestered growth factors, or in situ changes in mechanical properties, in response to an external stimulus, which then may alter cellular behaviour in real time. Although useful, these approaches are limited in that they are generally irreversible and do not truly establish a dynamic and mutual relationship between the cells and the biomaterial.
Living biomaterials offer a novel approach, in which incorporated bacteria imbue the material with organism-like properties, including the ability to become adaptive, productive and regenerative. In this study, the researchers expanded upon the living biomaterial concept, by providing a design in which the bacterial behaviour can be precisely controlled by light, thereby enabling manipulation of cell–material–bacteria interactions in real time.
The researchers genetically modified a strain of E. coli bacterium to display a cellular adhesion protein at its surface (the amino acid sequence RGD) upon stimulation with a drug (IPTG), which then enables the bacteria to interact with mammalian cells. In addition, they modified the bacteria to express red fluorescent protein (RFP) upon stimulation with IPTG, in order to identify activated bacteria. They then synthesized a photo-activatable version of IPTG (PA-IPTG), which would only activate the generation of the proteins upon light stimulation. Effects of light stimulation upon cellular behaviour. (a) Light induces cell–E. coli interactions. (b, c) MEFs only interact with E. coli that express RGD (E. coli+). (d) Focal adhesions are formed between MEFs and E. coli after light stimulation. e) No focal adhesions are formed when E. coli do not express RGD. (Courtesy: Advanced Science 10.1002/advs.201800383/CC BY 4.0)
Effects of light stimulation upon cellular behaviour. (a) Light induces cell–E. coli interactions. (b, c) MEFs only interact with E. coli that express RGD (E. coli+). (d) Focal adhesions are formed between MEFs and E. coli after light stimulation. e) No focal adhesions are formed when E. coli do not express RGD. (Courtesy: Advanced Science 10.1002/advs.201800383/CC BY 4.0)
 Effects of light stimulation upon cellular behaviour. (a) Light induces cell–E. coli interactions. (b, c) MEFs only interact with E. coli that express RGD (E. coli+). (d) Focal adhesions are formed between MEFs and E. coli after light stimulation. e) No focal adhesions are formed when E. coli do not express RGD. (Courtesy: Advanced Science 10.1002/advs.201800383/CC BY 4.0)
Effects of light stimulation upon cellular behaviour. (a) Light induces cell–E. coli interactions. (b, c) MEFs only interact with E. coli that express RGD (E. coli+). (d) Focal adhesions are formed between MEFs and E. coli after light stimulation. e) No focal adhesions are formed when E. coli do not express RGD. (Courtesy: Advanced Science 10.1002/advs.201800383/CC BY 4.0)
The team then seeded mouse embryonic fibroblasts (MEFs) onto the light-regulated living biomaterial and then tested how they could modulate MEF behaviour with light exposure. They found that without light, the MEFs did not interact with the bacteria and did not stretch out much across the material. Upon light exposure, the bacteria began to express RGD and RFP. The RGD sequence was recognised by MEF receptors, which enabled the formation of focal adhesions — the dynamic protein complexes that enable the cell cytoskeleton to interact with the environment. This formation of focal adhesions allowed the cells to fully stretch out and pick up the bacteria whilst migrating across the biomaterial.
Interestingly, the researchers also noted that the E. coli appeared to be secreting RFP over time, which was then taken up by the MEFs. This observation was unintentional but may potentially demonstrate a mechanism for the transfer of proteins from bacteria to cells within a living biomaterial.
This study provides an interesting proof-of-concept for a light-regulated living biomaterial. The team’s approach provides a simple method of inducing desired protein expression through light in E. coli. True reversibility and versatility however, may be obtained in the future through optogenetics technology. Optogenetics enables the use of light as a true “on–off” stimulus to induce a wide variety of cellular processes such as gene expression, cell signalling cascades and paracrine signalling. Dynamic Biomaterials group at the Leibniz Institute for New Materials.
Dynamic Biomaterials group at the Leibniz Institute for New Materials.
 Dynamic Biomaterials group at the Leibniz Institute for New Materials.
Dynamic Biomaterials group at the Leibniz Institute for New Materials.
It will be interesting to see whether future studies expand upon the work demonstrated here to further enhance the interactions of cells with biomaterials. Indeed, the researchers have already submitted extensions to this work in which they induce E. coli RFP secretion in a biomaterial through optogenetics (10.26434/chemrxiv.6852617.v1 and 10.26434/chemrxiv.7087010.v1).
18/9/2018 FROM PHYSICSWORLD.COM
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου