Kilogram finally redefined as world’s metrologists agree to new formulation for SI units
16 Nov 2018 Michael Banks
Metrologists and policy-makers from 60 countries around the world have unanimously agreed to change the definition of four units of measurement. At a meeting today at the General Conference on Weights and Measures (CGPM) in Versailles, France, delegates voted to redefine the International System of Units (SI), changing the world’s definition of the kilogram, the ampere, the kelvin and the mole. The changes will now come into force on 20 May 2019.
There are seven base units of the SI: the second, metre, kilogram, ampere, kelvin, mole and candela. Some have long been based on physical constants. The metre, for example, has been defined since 1983 as the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 seconds. But the four that metrologists today agreed to redefine were previously based on something – i.e. an object, experiment or phenomenon — meaning that its value is not universal.
New definitions
Today’s decision means that all seven SI units will now be defined in terms of physical constants. The biggest change will be to the kilogram, which is currently set by a 143-year-old platinum alloy cylinder, dubbed “Le Grand K” housed in the International Bureau of Weights and Measures (BIPM) in Paris. The kilogram will now be defined in terms of the Planck constant, h, which has been measured with extraordinary precision in recent years. Its agreed value will be set as 6.626 070 15 × 10–34 kg m2 s–1.
The ampere, meanwhile, will be set by the elementary electrical charge, e, which is given as 1.602 176 634 × 10–19 when expressed in coulombs. The Kelvin will be defined by taking the fixed numerical value of the Boltzmann constant k to be 1.380 649 × 10—23 when expressed in the unit J K-1 and the mole by the Avogadro constant (NA) contains exactly 6.02 214 076 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol–1.READ MORE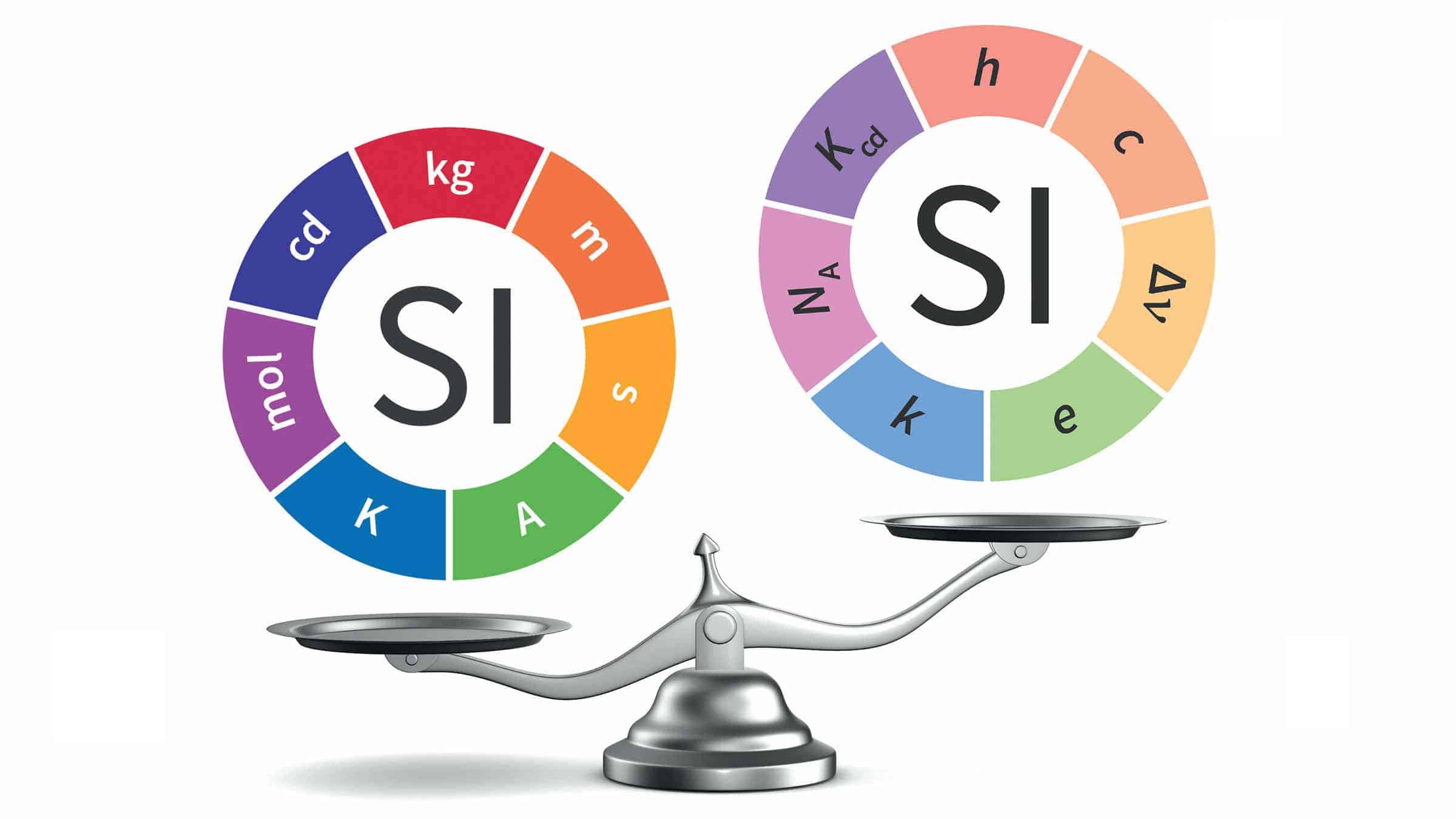

In daily life, however, the new SI units will have little immediate practical consequence. While the units may be defined differently, the goal has been to keep their size the same. Yet defining the units based on physical constants means that scientists will be able to measure them at any place or time, and on any scale. “The SI redefinition is a landmark moment in scientific measurement,” says Jan-Theodoor Janssen, director of research at the UK’s National Physical Laboratory. “This will pave the way for far more accurate measurements and lays a more stable foundation for science.”
16/11/2018 from physicsworld.com

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου