Microscopic materials hint at the origin of phagocytosis
A previously unidentified physically activated pathway for cellular uptake in the body’s immune defence system could predate commonly recognized receptor-mediated processes. The pathway relies on activation of the Moesin anchor protein through membrane deformation by physical objects, and is believed to have developed, at least 0.8 billion years ago. The researchers, a collaboration led by Tie Xia and Yan Shi at Tsinghua University in China and the University of Calgary in Canada, suggest that this ancient material-based pathway laid the groundwork for the evolution of immune receptor signalling.READ MORE

PIP2 lipids locate around physical objects
Phagocytosis is one of the main methods by which cells, in particular white blood cells, defend our body from external objects. Phagocytes, such as macrophages, neutrophils and others, rely on this process to physically ingest foreign particles, cellular debris and bacteria in our body and subsequently destroy and clear them from our system. In this work, Xia, Shi and colleagues incubated phagocytes with polysterene beads and deformed them with beads attached to an atomic force microscope (AFM) cantilever. When in contact, they observed clear localization of PIP2 lipids within the membrane around these objects. These lipids bind the Moesin anchor protein that activates phagocytosis.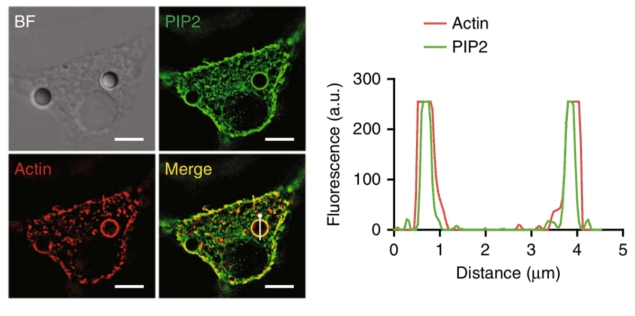 Phagocyte lipids surround polystyrene beads. Adapted from Mu et al/Nature Communications under Creative Commons Attribution 4.0
Phagocyte lipids surround polystyrene beads. Adapted from Mu et al/Nature Communications under Creative Commons Attribution 4.0
 Phagocyte lipids surround polystyrene beads. Adapted from Mu et al/Nature Communications under Creative Commons Attribution 4.0
Phagocyte lipids surround polystyrene beads. Adapted from Mu et al/Nature Communications under Creative Commons Attribution 4.0
Furthermore, by generating giant plasma membrane vesicles (GPMVs), the researchers were able to study how these lipids organize when in contact with topographical modified surfaces. By using micropatterned PDMS substrates consisting of square, circular and triangular pillars, the authors observed spontaneous localization of PIP2 lipids around these structures, suggesting that these lipids accumulate at deformed membrane regions in a purely physical manner without any cellular activity.
In addition, they showed that the stiffer the bead, the more efficient the phagocytosis. These results suggest key material properties for applications in microparticle drug delivery as well as how materials engineers can exploit cellular processes to deliver contents into cells.
Evolution from physical deformation
Finally, the authors knocked down the Moesin protein from their human phagocytes, disabling their phagocytotic behaviour, and then re-introduced it via transfection of the same protein cloned from fruit flies (Drosophilae Melanogaster), roundworms (C. Elegans) and zebrafish (Danio Rerio). These foreign proteins restored the cells’ phagocytotic response to physical deformation. Phylogenic analysis suggests that this protein has been conserved for over 797 million years as opposed to 435 million years for the first appearance of any protein with signaling potential (FcR γ chain) in FcR mediated phagocytosis. In fact, modern FcRs appeared only 90 million years ago. Hence, phagocytosis via physical membrane deformation by microscopic materials could be one of the original methods from which our immune systems developed.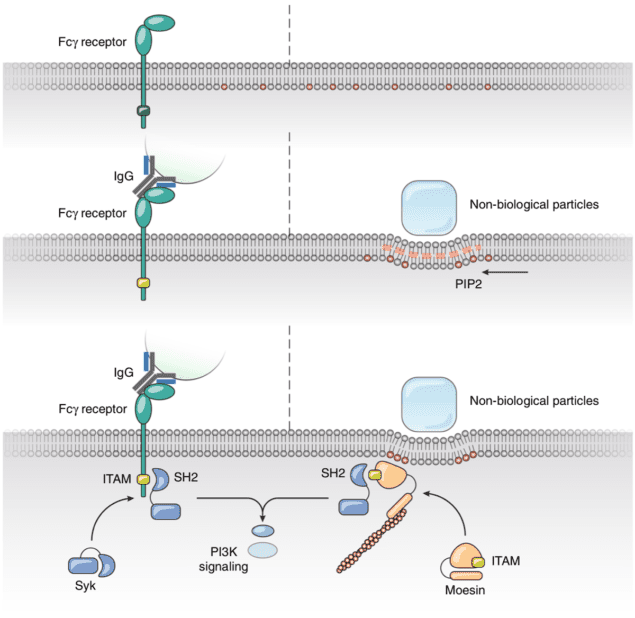 New versus old: ‘Modern’ receptor-based entry (left) as opposed to ‘ancient’ deformation-based entry (right) (Reproduced from Mu et al/Nature Communications under Creative Commons Attribution 4.0)
New versus old: ‘Modern’ receptor-based entry (left) as opposed to ‘ancient’ deformation-based entry (right) (Reproduced from Mu et al/Nature Communications under Creative Commons Attribution 4.0)
 New versus old: ‘Modern’ receptor-based entry (left) as opposed to ‘ancient’ deformation-based entry (right) (Reproduced from Mu et al/Nature Communications under Creative Commons Attribution 4.0)
New versus old: ‘Modern’ receptor-based entry (left) as opposed to ‘ancient’ deformation-based entry (right) (Reproduced from Mu et al/Nature Communications under Creative Commons Attribution 4.0)
In conclusion, these results have significant implications not only for the way we think about the origins of cellular functions but indeed on how we think about the cell-material interface.
Nanotechnology has led to many potential applications in the field of biology and medicine but many questions still remain unanswered as to how materials affect cells at the nanoscale. This work goes to show that, however modern our approach may be, cellular life is often a few hundred million years ahead of us.
Full details of the research are reported in Nature Communications
9/11/2018 FROM PHYSICSWORLD.COM

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου