How could galactic cosmic rays affect astronauts travelling to Mars?
07 Dec 2022 Tami Freeman
Future travel plans Artist's concept depicting astronauts and human habitats on Mars. (Courtesy: NASA)
With the pending return to long-duration crewed spaceflights, astronauts will face significant risks from exposure to space radiation. Galactic cosmic rays (GCRs) pose a particular challenge as they are not easily shielded and have dose rates as high as 0.5 mGy/day.
Sustained irradiation to the central nervous system is a major concern, both for long-term astronaut health and overall mission success. Studies in rodents have demonstrated behavioural changes following exposure to radiation doses as low as 50 mGy. Patients treated with radiotherapy have also experienced cognitive and memory impairments, albeit at much higher radiation doses. But accurate risk estimation for astronauts is difficult, in part due to the technical challenges of emulating the broad-spectrum GCR field in a laboratory.
In recent years, the NASA Space Radiation Laboratory has used a new GCR simulator (GCRSim) for its radiobiology experiments. The GCRSim spectrum includes 33 ion–energy combinations and closely resembles the radiation environment that astronauts will experience on journeys to the Moon and Mars.
Now a research team from Harvard University and Massachusetts General Hospital has performed the first nanometre-scale computational analysis of GCRSim in a realistic neuron geometry. The team hopes that the simulations, presented in Physics in Medicine & Biology, will help researchers performing GCRSim experiments interpret biological data.
“The motivation for this study was to simulate the energy deposition imparted to a neuron under realistic spaceflight conditions that can also be replicated during ground-based radiobiology experiments,” first author Jonah Peter tells Physics World.
Modelling the neuron
Radiation-induced behavioural changes are thought to arise partly from damage to neurons in the brain’s hippocampus. In particular, irradiation of sub-neuronal structures such as dendrites (branched extensions of the nerve cell) and dendritic spines (tiny protrusions from the dendrites) can cause cognitive decline. With this in mind, Peter and colleagues performed in silico reconstructions of a representative hippocampal neuron, including the soma (cell body), dendrites and over 3500 dendritic spines.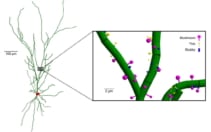 In silico reconstruction Neuron geometry showing the dendrites (green), soma (red) and a zoomed-in view of the dendritic spines. (Courtesy: Phys. Med. Biol. 10.1088/1361-6560/ac95f4)
In silico reconstruction Neuron geometry showing the dendrites (green), soma (red) and a zoomed-in view of the dendritic spines. (Courtesy: Phys. Med. Biol. 10.1088/1361-6560/ac95f4)
 In silico reconstruction Neuron geometry showing the dendrites (green), soma (red) and a zoomed-in view of the dendritic spines. (Courtesy: Phys. Med. Biol. 10.1088/1361-6560/ac95f4)
In silico reconstruction Neuron geometry showing the dendrites (green), soma (red) and a zoomed-in view of the dendritic spines. (Courtesy: Phys. Med. Biol. 10.1088/1361-6560/ac95f4)The team used Monte Carlo simulations to model particle tracks through the neuron for each GCRSim ion–energy combination, which included 14 different energies of protons and alpha particles, plus five heavier ions.
For all simulations, the total absorbed dose over the entire neuron was scaled to 0.5 Gy, the approximate dose experienced by an astronaut during a 2–3 year Mars mission, and the dose used in GCRSim experiments.
The model predicted absorbed doses to the soma, dendrites and spines after GCRSim irradiation of 0.54±0.09, 0.47±0.02 and 0.8±0.5 Gy, respectively – deviating from 0.5 Gy due to inhomogeneities in the irradiation profile at low fluence. “This leads to stochastic fluctuations in the absorbed dose, which become more prominent for smaller structures,” Peter explains.
The researchers also analysed the energy deposition for three dendritic spine types (mushroom, thin and stubby spines). They found that mushroom spines receive around 78% of the total spine energy deposition due to their larger average volume, which could put them at greater risk for radiation-induced damage. Dose predictions Doses to the neuron and subcellular structures after GCRSim irradiation, scaled to a total neuronal absorbed dose of 0.5 Gy. (Courtesy: J S Peter et al Phys. Med. Biol. 10.1088/1361-6560/ac95f4)
Dose predictions Doses to the neuron and subcellular structures after GCRSim irradiation, scaled to a total neuronal absorbed dose of 0.5 Gy. (Courtesy: J S Peter et al Phys. Med. Biol. 10.1088/1361-6560/ac95f4)
 Dose predictions Doses to the neuron and subcellular structures after GCRSim irradiation, scaled to a total neuronal absorbed dose of 0.5 Gy. (Courtesy: J S Peter et al Phys. Med. Biol. 10.1088/1361-6560/ac95f4)
Dose predictions Doses to the neuron and subcellular structures after GCRSim irradiation, scaled to a total neuronal absorbed dose of 0.5 Gy. (Courtesy: J S Peter et al Phys. Med. Biol. 10.1088/1361-6560/ac95f4)Energy deposition
Due to the high energies of all primary ions in the GCRSim spectrum, each ion deposits most of its energy into the neuron via secondary electrons. The team investigated the various physical processes associated with this energy deposition and found that the dominant contribution (59%) came from ionizations. This is significant, as ionizations inflict the largest energy deposition per event, making them particularly harmful.
For a GCRSim neuron dose of 0.5 Gy, the simulations predicted an average of 1760±90 energy deposition events per micrometre of dendritic length, 250±10 of which were ionizations. In addition, there were an average of 330±80, 50±20 and 30±10 events per mushroom, thin and stubby spine, respectively, including 50±10, 7±2 and 4±2 ionizations per spine.
Assessing the spatial distribution of energy deposition events throughout the dendrites revealed that GCRSim exposure results in proton irradiation of all dendritic segments at very low doses. Widespread irradiation by alpha particles was also likely at spaceflight-relevant doses, while irradiation by heavier ions was comparatively rare.
“There is still a lot of uncertainty surrounding which aspects of GCR irradiation are ultimately responsible for eventual changes in cognition or behaviour,” Peter explains. “Our results suggest that widespread irradiation of even small-scale structures like neuronal dendrites is likely after just a few months of spaceflight.”
If such repeated, widespread irradiation is indeed the driver of neuronal dysfunction, this might imply that extended deep-space missions are disproportionately more dangerous than short stays in low Earth orbit. Peter notes that more experimental data are needed, however, before any definitive conclusions can be drawn.READ MORE

Finally, the researchers compared their results to those obtained using SimGCRSim, a simplified spectrum also employed in NASA experiments. They found that the 33-beam GCRSim and the 6-beam SimGCRSim irradiation profiles produced highly similar fluences and energy deposition patterns at the single-neuron scale.
The ultimate goal, says Peter, is to develop a mechanistic model of radiation-induced neuronal dysfunction. The team’s next step will be to include the effects of radiolytic chemistry in the simulations and then, when more experimental data are available, to deduce which physico-chemical properties are responsible for changes in biological function.

Tami Freeman is an online editor for Physics World
from physicsworld.com 7/12/2022

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου