In vitro platform enables realistic studies of neurological disorders
04 Feb 2022 Tami Freeman

Pre-clinical research into neurological disorders is limited by the complexity of the brain, as well as the lack of adequate models to assess potential drugs, as demonstrated by the discouraging failure of drug candidates in late stages of clinical trials. Two-dimensional cell cultures can mimic some aspects of neuronal dysfunction, but lack complex morphology.
Animal models, meanwhile, are often not sufficiently relevant for studying the human brain.
Three-dimensional cell models, so-called microphysiological systems (MPS), can more accurately mimic the structure and function of neural tissue. However, recording the electrical activity of individual neurons – the most relevant measure of neuronal function – remains challenging in 3D neuronal structures.
“A neuro-relevant in vitro 3D system capable of assessing the efficacy or toxicity of substances by measuring neuronal activity is still missing,” explains Paolo Cesare from the NMI Natural and Medical Sciences Institute in Reutlingen. “This is mainly because of the technical challenge of integrating a direct readout of electrical activity with millisecond resolution and high sensitivity.”
To fill this gap, Cesare and colleagues have developed a novel neuro-MPS that provides continuous, non-invasive electrophysiological readout of 3D neuronal networks.
Device design
The neuro-MPS, described in Biofabrication, is based on a multiwell glass microfluidic device. Each well contains a 3D hydrogel layer, into which dissociated brain cells are dispersed, covered by a liquid layer containing culture medium. Microelectrodes integrated into the base of each well record the electrical activity from the neurons in the hydrogel.
The key innovation, say the researchers, is the introduction of capped microelectrodes (CMEs), which are insulated with a thin cap containing 5 µm-wide tunnels. As the embedded neurons mature, they extend projections called neurites (axons or dendrites) in all directions, including into the CME tunnels. When the neurons fire, electrical signals travel along these neurites and into the caps, generating action potentials of up to hundreds of microvolts.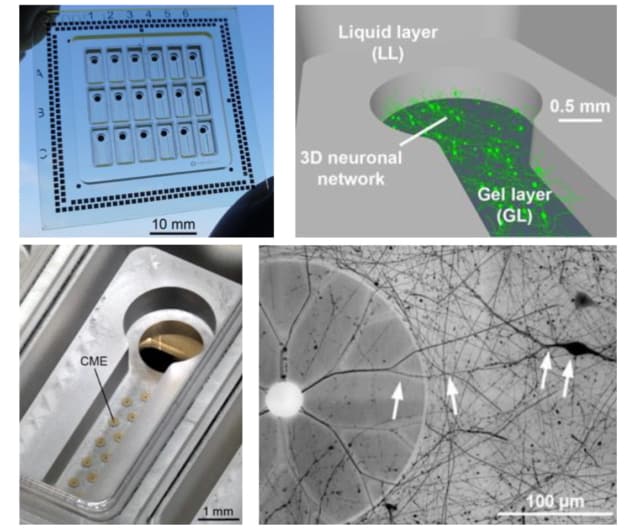 Culturing 3D brain cells: A neuro-MPS with 18 independent wells (top left); neurons embedded in a 3D scaffold within the hydrogel layer (top right); CMEs in the base of a well (bottom left); a neuron embedded in hydrogel extends neurites into the CME tunnels (bottom right). (Courtesy: Biofabrication 10.1088/1758-5090/ac463b)
Culturing 3D brain cells: A neuro-MPS with 18 independent wells (top left); neurons embedded in a 3D scaffold within the hydrogel layer (top right); CMEs in the base of a well (bottom left); a neuron embedded in hydrogel extends neurites into the CME tunnels (bottom right). (Courtesy: Biofabrication 10.1088/1758-5090/ac463b)
 Culturing 3D brain cells: A neuro-MPS with 18 independent wells (top left); neurons embedded in a 3D scaffold within the hydrogel layer (top right); CMEs in the base of a well (bottom left); a neuron embedded in hydrogel extends neurites into the CME tunnels (bottom right). (Courtesy: Biofabrication 10.1088/1758-5090/ac463b)
Culturing 3D brain cells: A neuro-MPS with 18 independent wells (top left); neurons embedded in a 3D scaffold within the hydrogel layer (top right); CMEs in the base of a well (bottom left); a neuron embedded in hydrogel extends neurites into the CME tunnels (bottom right). (Courtesy: Biofabrication 10.1088/1758-5090/ac463b)“The core technology is inspired by nature, in particular by the biological process of nerve sheathing through myelin, a thin membrane that electrically isolates axons and contributes to the rapid transmission of electrical signals in the nervous system,” says Cesare. “This process has been replicated at NMI by using different technologies, processes and materials to produce insulated microelectrodes that are able to measure the tiny electrical signals that propagate along the axons in 3D neuronal cultures.”
The researchers used various CME configurations to record the electrical activity of 3D-cultured primary mouse hippocampal neurons. They found that having more tunnels in each cap increased ease of access for neurites and thus the mean firing rate, but decreased the signal-to-noise ratio. Open electrodes with no cap detected negligible action potentials, showing their unsuitability for recording from neurons in a 3D hydrogel.
For the neuro-MPS to provide representative in vitro studies, it’s important that the individual neurons form a functional 3D network. The synchronized action potentials recorded by the CMEs suggest that the neurons are synaptically connected. But to confirm that the substrate-integrated CMEs aren’t preferentially recording from neurons near the bottom of the well, the researchers performed electrical recordings simultaneously with calcium imaging to identify active neurons. Calcium imaging measured activity at least 500 µm above the electrodes and the CMEs recorded concurrent activity, indicating that they are recording from throughout the entire 3D neuronal network.
Next, the team quantified the response to picrotoxin (PTX) and tetrodotoxin (TTX), which increase and reduce neuronal network activity, respectively. Low PTX concentrations significantly affected network activity, increasing the mean firing rate and the mean firing rate in network bursts, and decreasing the network burst frequency. TTX at higher concentrations significantly reduced neuronal activity.
The researchers also investigated optogenetic stimulation in the neuro-MPS, using neurons that react to pulsed 470 nm light. They illuminated a region in the 3D culture with light pulses and recorded the electrical activity of the entire neuronal network. Optical activation of neurons in the illuminated area triggered synchronized network bursts across the entire well, which were measured by individual CMEs. The team suggest that such optogenetic stimulation could provide a new approach for studying learning and memory in vitro.
Multimodal analysis
Another feature of the neuro-MPS is its ability to combine functional and structural analysis, as its transparent glass base enables confocal microscopy of the 3D neuronal structures. The team demonstrated this by examining the effects of rotenone – a pesticide that creates alterations associated with Parkinson’s disease – on mature neuronal cultures.
The CMEs recorded changes in neuronal activity just 10 min after exposure to 0.05 µM rotenone. Morphological changes assessed via live-cell imaging, however, appeared only 6 and 24 h after doses of 5 and 0.5 µM, respectively. This demonstrates that electrophysiological assessment can supplement structural studies and provide a more sensitive way to study the effects of neuroactive and toxic compounds.READ MORE

“Mimicking cell composition and structure of the nervous system, while providing a high throughput platform for non-invasive acquisition of structural and electrophysiological data from 3D neural networks, represents a major leap forward towards a more physiological and brain relevant preclinical research platform,” Cesare tells Physics World.
Cesare and colleagues are now modifying the technology to create a new MPS that measures electrical activity in self-aggregated brain tissues, such as 3D brain organoids. They also hope to expand application of the MPS to the peripheral nervous system, creating devices to study 3D innervation of different target tissues.

Tami Freeman is an online editor for Physics World
from physocsworld.com 5/2/2022
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου